LABORATORIES
Direct neuronal reprogramming laboratory
Direct neuronal reprogramming laboratory
Research
Team
Publications
Contact
Others
Research

Lines of investigation
1) Use of hypothermia to favor the reprogramming of glial cells to induced neurons
In a previous study, we demonstrated that there is a metabolic barrier that prevents the reprogramming of various cell types to neurons (Gascón et al., 2016, 2017). This barrier has its origin in the metabolic transition, from glycolysis to oxidative phosphorylation, that cells experience during neuronal conversion, which leads to the formation of free oxygen radicals and cell death by ferroptosis.
The starting hypothesis of this project is that the reduction in the speed at which chemical reactions occur during neuronal reprogramming, induced by hypothermia, provides the necessary time for cells to adapt to the new metabolic identity, which decreases the formation of free oxygen radicals. Our data indicate that, under hypothermic conditions, various types of glial cells in culture can be reprogrammed into neurons more efficiently. The ultimate goal of this line of work is to apply hypothermia in in vivo neuronal reprogramming models to increase efficiency.
of therapies based on cell replacement.
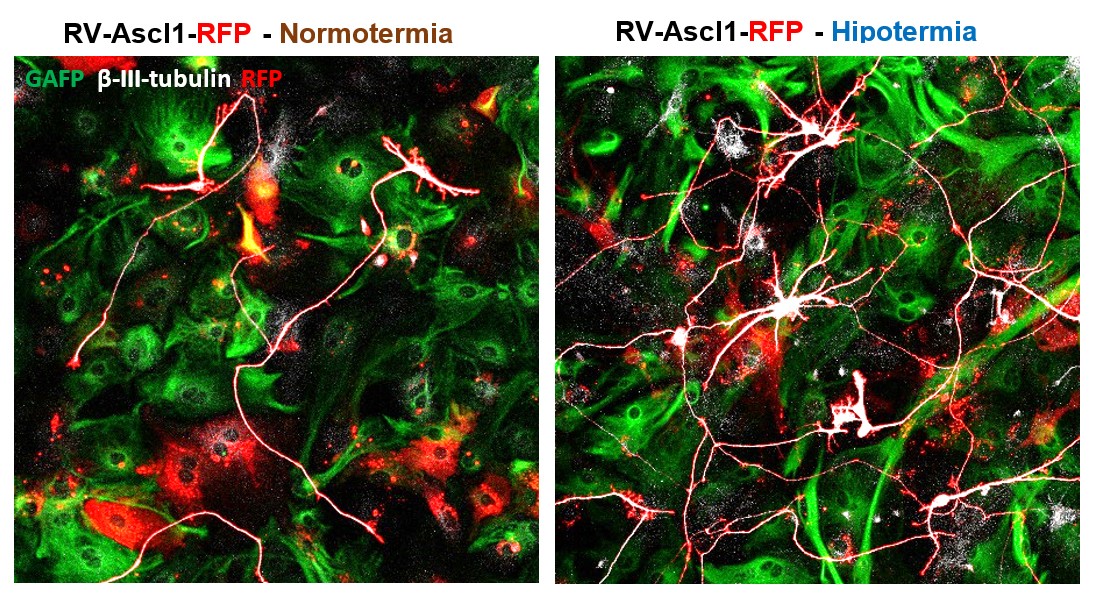
The image on the left shows iNs derived from reprogrammed mouse astrocytes under normothermic conditions,
while the figure on the right shows reprogrammed cells under hypothermic conditions.
2) Identification of epigenetic barriers that make it difficult to obtain specific iNs subtypes.
One of the biggest challenges in the field of direct neural reprogramming is the generation of all the neuron subtypes that make up the brain. This is exceptionally complex due to the wide variety of existing neuronal subtypes and the lack of knowledge of the transcriptomic programs that determine the acquisition of specific neuronal identities. In this sense, it is thought that the epigenetic changes that take place during neuronal differentiation could be determinant both for the acquisition of specific neuronal identities and to prevent different neuronal subtypes, already established, from spontaneously reversing their identity. This hypothesis is supported by the fact that while young neurons or neuroblasts can be reprogrammed into alternative neuronal subtypes in response to overexpression of certain lineage selector genes, no strategy is known to change the identity of neurons once that these are ripe. Although these epigenetic restrictions are fundamental for the correct development and functioning of the brain, they are a major obstacle in obtaining iNs that belong to neuronal subpopulations of interest to the scientific community, and that may be relevant in regenerative therapies based on cell replacement. Therefore, this line of research aims to discover new epigenetic mechanisms that underlie the specification of neuronal subtypes, in models of neurogenesis and neuronal reprogramming in the adult brain to advance in the understanding of the molecular bases that regulate these processes.
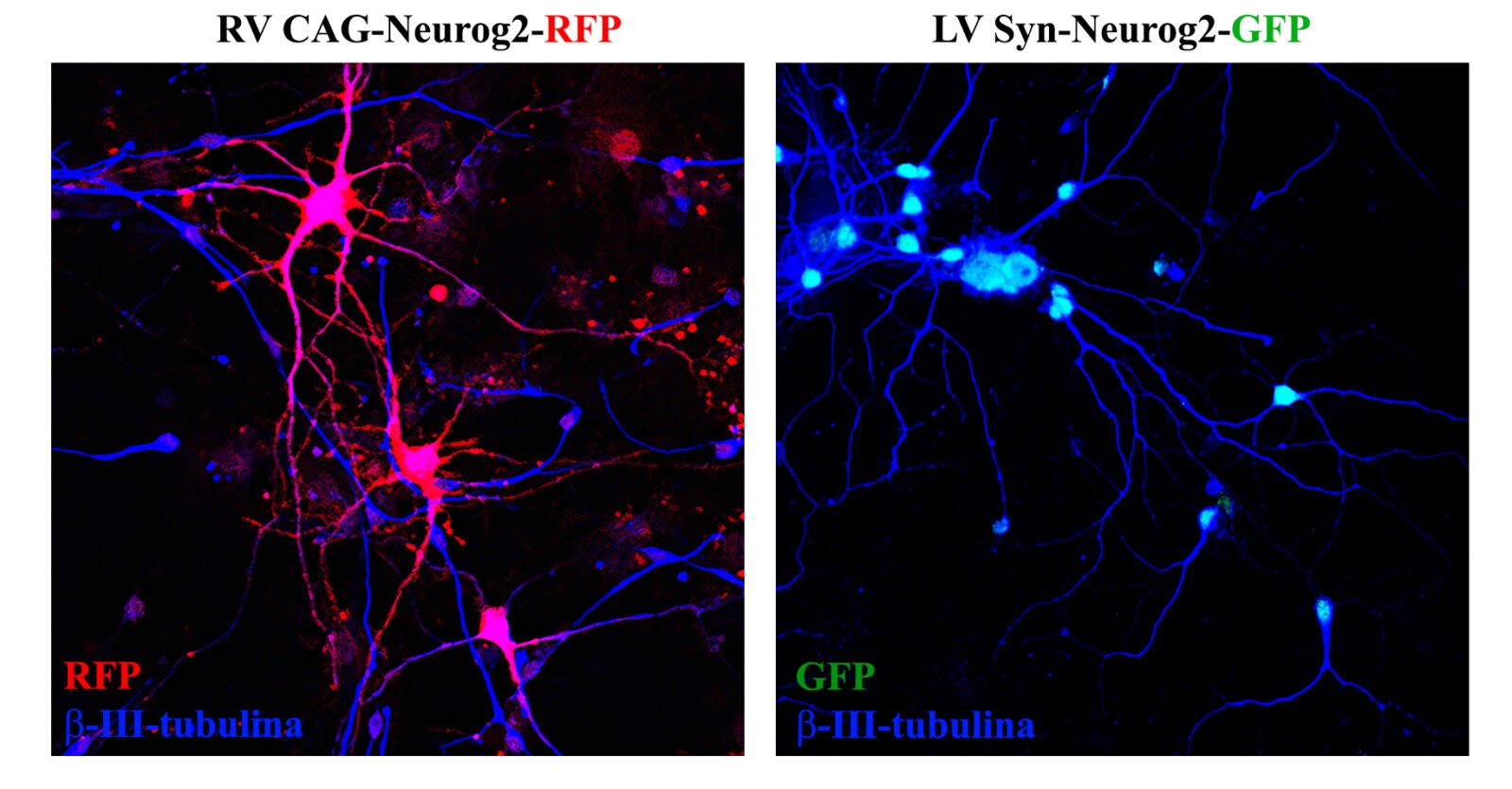
Shown on the left are cells derived from the mouse subventricular zone (originally of lineage
GABAergic), respecified to glutamatergic neurons (note the large soma and the great complexity
dendritic) by the retroviral expression of the neurogenic factor Neurog2. On the right it can be seen that the
lentiviral expression of the same factor has no effect on already differentiated GABAergic neurons.
3) Direct neural reprogramming for the study of neurodegenerative diseases.
In recent decades, iPSCs have been the most appropriate starting material to obtain and study different types of human cells in vitro. However, the acquisition of pluripotency leads to cell rejuvenation, which makes it difficult to study the molecular and/or cellular correlations between neurodegenerative diseases and the cellular aging process. To solve this, a promising alternative is the use of methodologies based on direct cell conversion, which maintains the initial age of the reprogrammed cells.
In our laboratory, we use this approach to reprogram human dermal fibroblasts into different types of neurons and other cells relevant to CNS diseases, such as motor neurons and skeletal muscle myotubes, two lineages affected in amyotrophic lateral sclerosis (ALS). In this case, for neuronal reprogramming we use retroviral vectors that induce the expression of the Neurog2, Isl1 and Bcl-2 genes. The combination of these genes, both in control fibroblasts and in those from ALS patients, gives rise to a pure population of induced motor neurons (iMNs, β-III-tubulin + , Hb9 + and Isl2 + ). To obtain skeletal muscle, we used a similar methodology, based on the retroviral expression of the myogenic factor MyoD. Under specific culture conditions, induced myotubes (iMs) are immunoreactive for phalloidin and myosin, display well-defined sarcomeric structures, and establish neuromuscular junctions (detectable with bungarotoxin) with iMNs.
These types of cultures are an ideal platform for the development of different models of neurodegenerative diseases, and our team uses them extensively in the study of human pathology.
More information in www.gasconlab.com
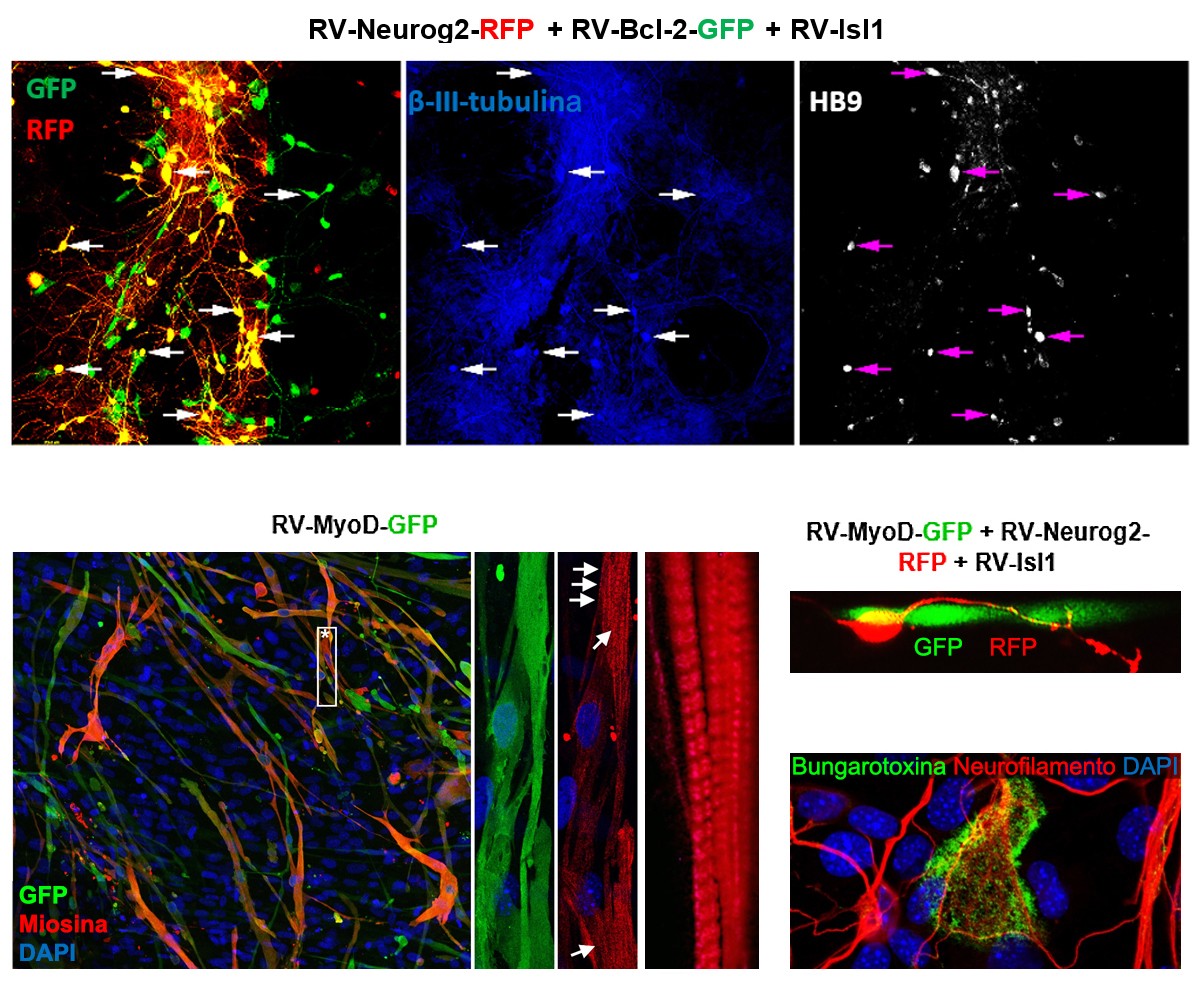
The top images show iMNs derived from reprogrammed human fibroblasts. The
lower images show iMs in monoculture (left) or cocultured with iMNs (right)
derived from reprogrammed human fibroblasts. In the cocultures, the presence of
neuromuscular junctions detected by bungarotoxin.
Most relevant methodology
1) Development of primary cultures of mouse cells and human lines, as well as their use in cell reprogramming models.
2) Methods of analysis of the cellular redox state and oxidative stress during neuronal reprogramming.
3) Neuronal reprogramming techniques in the adult mouse brain.
4) Production and use of retroviral vectors for the expression of exogenous proteins.
5) Use of rabies virus for retrograde synaptic scanning.
6) Molecular biology and cell biology techniques, such as cloning, immunocytochemistry, immunohistochemistry, flow cytometry and profile analysis
single cell transcriptomic
Team
Former members participating in ongoing research projects
Dulce María Arzate
Postdoctoral researcher
Ana Victoria Prádanos Senén
TFM student (nowadays, PhD student)
Publications
Publications
Patents:
1. Gascón S and Götz M. Trans-differentiation of differentiated cells. European patent application for anti-oxidant molecules in direct neuronal reprogramming, WO 2015/114059 A1. 2015.
Book chapters:
2. Falco A, Bartolomé-Cabrero R, Gascón S. Bcl-2-Assisted Reprogramming of Mouse Astrocytes and Human Fibroblasts into Induced Neurons. Methods Mol Biol. 2021;2352:57- 71. doi: 10.1007/978-1-0716-1601-7_5. PMID: 34324180.
3. Lucía Paniagua-Herranz, Rosa Gómez-Villafuertes, David de Agustín-Durán, Sergio Gascón, Raquel Pérez-Sen, Esmerilda G. Delicado, María Teresa Miras-Portugal, Felipe Ortega. Time-Lapse Video Microscopy and Single Cell Tracking to Study Neural Cell Behavior In Vitro. Methods in Molecular Biology 2020;2150:183-194. doi: 10.1007/7651_2019_219.
Scientific reviews:
4. Stockwell BR, Friedmann Angeli JP, Bayir H, Bush A, Conrad M, Dixon S, Fulda S, Gascón S, Hatzios S, Kagan V, Noel K, Jiang X, Linkermann A, Murphy M, Overholtzer M, Oyagi A, Pagnussat G, Park J, Prives C, Ran Q, Rosenfeld C, Salnikow K, Tang D, Torti F, Torti S, Toyokuni S, Woerpel K, Zhang D. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017. Oct 5;171(2):273-285. doi: 10.1016/j.cell.2017.09.021.
5. Gascón S*, Masserdotti G*, Russo G*, Götz M. Direct neuronal reprogramming – achievements, hurdles and new roads to success. Cell Stem Cell. 2017 Jul 6;21(1):18-34. doi: 10.1016/j.stem.2017.06.011.
6. Masserdotti G*, Gascón S*, Götz M. Direct neuronal reprogramming-Learning from and for development. Development. 2016 143: 2494-2510; doi: 10.1242/dev.092163.
Original articles:
7. Peron S, Brill M, Miyakoshi L, Ortega F, Manzano D, Gascón S, Berninger B. Programming of neural progenitors of the adult subependymal zone towards a glutamatergic identity by Neurog2. (Stem Cell Reports (IF7.8), in advanced revision). Preliminary version already published at:
http://www.biorxiv.org/content/early/2017/08/03/171686.full.pdf+html
8. Karakatsani A, Marichal N*, Urban, S*, Kalamakis G, Schick A, Zhang Y, Ghanem A, Zhang, Y., Conzelmann K, Rüegg MA, Berninger B, Ruiz de Almodovar C, Gascón S, Kröger. Neuronal LRP4 Regulates Synapse Formation in the Developing CNS. Development. 2017 Oct 23. pii: dev.150110. doi: 10.1242/dev.150110.
9. Gómez-Villafuertes R*, Paniagua-Herranz L*, Gascón S*, Agustín-Durán D, Ferreras M, Gil-Redondo JC, Queipo MJ, Menendez-Mendez A, Pérez-Sen R, Delicado EG, Gualix J, Costa MR, Schroeder T, Miras-Portugal MT, Ortega F. Live imaging followed by single cell tracking to monitor cell biology and the lineage progression of multiple neural populations. J Vis Exp. 2017 Dec 16;(130). doi: 10.3791/56291.
10. Gascón Sɸ, Ortega F, Götz Mɸ. Transient CREB-mediated transcription is key in direct neuronal reprogramming. Neurogenesis. 2017 Feb 6;4(1):e1285383. doi: 10.1080/23262133.2017.1285383.
11. Gascón Sɸ*, Murenu E*, Masserdotti G, Ortega F, Russo G, Petrik D, Deshpande A, Heinrich C, Karow M, Robertson S, Schroeder, Beckers J, Irmler M, Berndt C, Friedmann JP, Conrad M, Berninger B, Götz M. Identification and successful negotiation of a metabolic checkpoint in direct neuronal
reprogramming. Cell Stem Cell. 2016 Mar 3;18(3):396-409. doi: 10.1016/j.stem.2015.12.003 This work has been highlighted by F1000Prime as being of special significance in its field. https://f1000.com/prime/726066295?subscriptioncode=3248f6ce-490a-498d-963a-b4374a3300a9 [See also Previews, by Quadrato G, Zhang AC, Arlotta P (2016) Stressed out? Healing tips for newly reprogrammed neurons, Cell Stem Cell 18: 297-299].
12. Sirko, S, Irmler M, Gascón S, Bek S, Schneider S, Dimou L, Obermann J, De Souza Paiva D, Poirier F, Beckers J, Hauck SM, Barde YA, Götz M. Astrocyte reactivity after brain injury: The role of galectins 1 and 3. Glia. 2015 Dec;63(12):2340-61. doi: 10.1002/glia.22898
13. Faiz M, Sachewsky N, Gascón S, Bang KW, Morshead CM, Nagy A. Adult Neural Stem Cells from the Subventricular Zone Give Rise to Reactive Astrocytes in the Cortex after Stroke. Cell Stem Cell. 2015 Nov 5;17(5):624-34. doi: 10.1016/j.stem.2015.08.002
14. Heinrich C*, Bergami M*, Gascón S, Lepier A, Viganò F, Dimou L, Sutor B, Berninger B, Götz M. Sox2-mediated conversion of NG2 glia into induced neurons in the injured adult cerebral cortex. Stem Cell Reports. 2014 Dec 9;3(6):1000-14. doi: 10.1016/j.stemcr.2014.10.007
15. Ortega F, Gascón S*, Masserdotti G*, Deshpande A, Simon C, Dimou L, Lie C, Schroeder T, Berninger B. Oligodendrogliogenic and neurogenic adult subependymal zone neural stem cells constitute distinct lineages and exhibit differential responsiveness to Wnt signalling. Nature Cell Biology. 2013 Jun;15(6):602-13. doi: 10.1038/ncb2736
16. Karow M*, Sánchez R*, Schichor, C, Masserdotti G, Ortega F, Heinrich C, Gascón S, Kahn A, Lie C, Dellavalle A, Cossu G, Goldb-runner R, Götz M, Berninger B. Reprogramming of pericyte-derived cells of the adult human brain into induced neuronal cells. Cell Stem Cell. 2012 Oct 5;11(4):471-6. doi: 10.1016/j.stem.2012.07.007
17. Vidaurre OG*, Gascón S*, Deogracias R, Sobrado M, Cuadrado E, Montaner J, Rodríguez-Peña A, Diaz-Guerra M. Imbalance of neurotrophin receptor isoforms TrkB-FL/TrkB-T1 induces neuronal death in excitotoxicity. Cell Death & Disease. 2012 Jan 19;3:e256. doi: 10.1038/cddis.2011.143
18. Heinrich C, Gascón S, Masserdotti G, Lepier A, Sanchez R, Simon-Ebert T, Schroeder T, Götz M, Berninger B.Generation of subtype-specific neurons from postnatal astroglia of the mouse cerebral cortex. Nature Protocols. 2011 Feb;6(2):214-28. doi: 10.1038/nprot.2010.188
19. Heinrich C, Blum R*, Gascón S*, Masserdotti G*, Tripathi P, Sánchez R, Tiedt S, Schroeder T, Götz M, Berninger B. Directing astroglia from the cerebral cortex into subtype specific functional neurons. PLoS Biology. 2010 May 18;8(5):e1000373. doi: 10.1371/journal.pbio.1000373
20. Brill MS*, Ninkovic J*, Winpenny E*, Hodge RD*, Ozen I, Yang R, Lepier A, Gascón S, Erdelyi F, Szabo G, Parras C, Guillemot F, Frotscher M, Berninger B, Hevner RF, Raineteau O, Götz M. Adult generation of glutamatergic olfactory bulb interneurons. Nature Neuroscience. 2009 Dec;12(12):1524-33. doi: 10.1038/nn.2416
21. López-Menéndez C, Gascón S, Sobrado M, Vidaurre OG, Higuero AM, Rodríguez-Peña A, Iglesias T, Díaz-Guerra M. Kidins220/ARMS downregulation by excitotoxic activation of NMDARs reveals its involvement in neuronal survival and death pathways. Journal of Cell Science. 2009 Oct 1;122(Pt 19):3554-65. doi: 10.1242/jcs.056473
22. Gascón S, Paez-Gomez JA, Díaz-Guerra M, Scheiffele P, Scholl FG. Dual-promoter lentiviral vectors for constitutive and regulated gene expression in neurons. Journal of Neuroscience Methods. 2008 Feb 15;168(1):104-12. doi: 10.1016/j.jneumeth.2007.09.023
23. Gascón S, Sobrado M, Roda JM, Rodríguez-Peña A, Díaz-Guerra M. Excitotoxicity and focal cerebral ischemia induce truncation of the NR2A and NR2B subunits of the NMDA receptor and cleavage of the scaffolding protein PSD-95. Molecular Psychiatry. 2008 Jan;13(1):99-114. We provided the cover in this issue. doi: 10.1038/sj.mp.4002017
24. Gascón S*, García-Gallo M*, Renart J, Díaz-Guerra M. Endoplasmic reticulum-associated degradation of the NR1 but not the NR2 subunits of the
N-methyl-D-aspartate receptor induced by inhibition of the N-glycosylation in cortical neurons. Journal of Neuroscience Research. 2007 Jun;85(8):1713-23. doi: 10.1002/jnr.21309
25. Gascón S, Deogracias R, Sobrado M, Roda JM, Renart J, Rodríguez-Peña A, Díaz- Guerra M. Transcription of the NR1 subunit of the N-methyl-D-aspartate receptor is down-regulated by excitotoxic stimulation and cerebral ischemia. The Journal of Biological Chemistry. 2005 Oct 14;280(41):35018-27. doi: 10.1074/jbc.M504108200
Contacto
Where to find us
Neural reprogramming lab
Instituto Cajal CSIC. Avda. Doctor Arce, 37. 28002. Madrid
Call us
Phone:
Write us
Email address:
Others
Collaborations
-
Ana Martínez Gil. Centro de Investigaciones Biológicas.
-
Margarita Salas. España.
-
Alicia González Martín. Instituto de Investigaciones Biomédicas “Alberto Sols”. España.
-
Benedikt Berninger. Centre for Developmental Neurobiology, King´s College of London. Reino Unido.
-
Christophe Heinrich. Stem Cell and Brain Research Institute – INSERM, Lyon. Francia.
-
Dora Brites. Faculty of Pharmacy, University of Lisbon, Lisbon, Portugal.
-
Eva de Lago Femia. Facultad de Medicina. Universidad Complutense de Madrid. España.
-
Felipe Ortega. Facultad de Veterinaria, Universidad Complutense de Madrid. España
-
Fernando de Castro Soubriet. Departamento de Neurobiología Celular, Molecular y del Desarrollo. Instituto Cajal (CSIC). España.
- Francisco Manuel Gómez Scholl. Departamento de Fisiología Médica y Biofísica, IBiS. Facultad de Medicina. Universidad de Sevilla. España.
- José Luis Trejo Pérez. Departamento. Departamento de Neurociencia Traslacional. Instituto Cajal (CSIC). España.
-
Laura López Mascaraque. Departamento de Neurobiología Celular, Molecular y del Desarrollo. Instituto Cajal (CSIC). España.
-
Magdalena Götz. Ludwig-Maximilians-University of Munich. Alemania.
-
María del Valle Palomo Ruiz. Instituto Madrileño de Estudios Avanzados. España.
-
Toni del Río. Instituto Biotecnología de Cataluña (IBEC). Barcelona. España.
Funding
- Scientific projects.
1) Project “Role of the epigenetic regulator Ring1B in determining the neuronal subtype during adult neurogenesis and neuronal reprogramming”, Knowledge Generation Projects 2021. Ministry of Science and Innovation. (PID2021-128796OB-I00). 01/01/2022 – 12/31/2024.
2) Project “Targeting TDP-43 with protein kinase inhibitors: an effective and measurable therapy for ALS?” La Caixa Foundation (LCF/PR/HA21/52350003; CSIC Consortium, FARM-ID, UCM and IMDEA). 11/1/2021 –11/1/2024.
3) Project “Analysis of Synaptic Connectivity between Induced Neurons” (Ref. 202020E061). Cajal Institute. From 01/15/2020.
4) Project “Role of infiltrated myeloid cells and reactive macroglia in direct neuronal reprogramming of mouse and human cells”. National research program oriented to the challenges of society. Ministry of Science, Innovation and Universities (RTI2018-099345-B-I00). 01/01/2019 – 09/01/2022.
5) Project “Modeling ALS disease in vitro”-MAIV 01EK1611A. Federal Ministry of Education and Research (BMBF-Germany). 02/01/2017 to 02/01/2022.
6) Project – help Ramon & Cajal. Ministry of Science, Innovation and Universities (RYC-2015-19185). 08/31/2017 – 08/30/2022. - Funding obtained by students/researchers associated with the research team.
1) Postdoctoral fellowship CONACyT (National Council of Science and Technology, Mexico) of Dr. Dulce María Arzate. 09/30/2020 to 09/30/2022.
2) JAE Intro Scholarship – CSIC for Ana Victoria Prádanos Senén. 2021-2022.
3) Contract “Youth Grant” (Community of Madrid) of Víctor Álvaro Sánchez. 05/01/2021 – 05/01/2023.

Neuroscience Research Center dependent on the CSIC. Founded in 1920 and initially directed by Santiago Ramón y Cajal. World reference in the study of the brain. Custodian of the Cajal Legacy.
Activities








