LABORATORIES
Neurophysiology and Synaptic Plasticity
Neurophysiology and Synaptic Plasticity
Research
Team
Publications
Contact
Others
Research
Our group is focused in the study of the cellular processes and molecular mechanisms underlying the regulation of synaptic transmission. We are particularly interested in the ability of neurons to regulate the synaptic strength over time, a process called synaptic plasticity, which is crucial both during physiological and pathological conditions of the Nervous System (NS).

Lines of investigation
Our goal is to investigate the properties, mechanisms and consequences of astrocyte signaling on neuronal excitability and synaptic transmission in the control of motor activity. The general aim of our work is elucidate whether activation of astrocytes regulates potassium conductances, glutamatergic and GABAergic synaptic transmission and, consequently, modulates motor network excitability in physiological and pathological conditions. We propose a multidisciplinary approach to study different aspects of neuron-astrocytes interaction, from changes in intracellular calcium signaling, effects on synaptic transmission, remodeling of synaptic structures, changes in intrinsic neuronal properties, excitability, integration of information into neural circuits and, finally, the consequences on behavior.
In our studies, we apply several functional and structural approaches to the experimental animal models, including:
- Extra and intracellular (patch- clamp technics) recordings of neuronal electrical activity.
- Simultaneous recordings of electrical activity and calcium imaging both in brain slices and in vivo.
- Electrochemistry techniques (fast cyclic voltammetry at the carbon fiber microelectrode, amperometry, biosensors).
- Optogenetic and chemogenic stimulation (ex vivo and in vivo).
- Electrophysiology, calcium imaging and behavioral tests combined in freely moving animals.
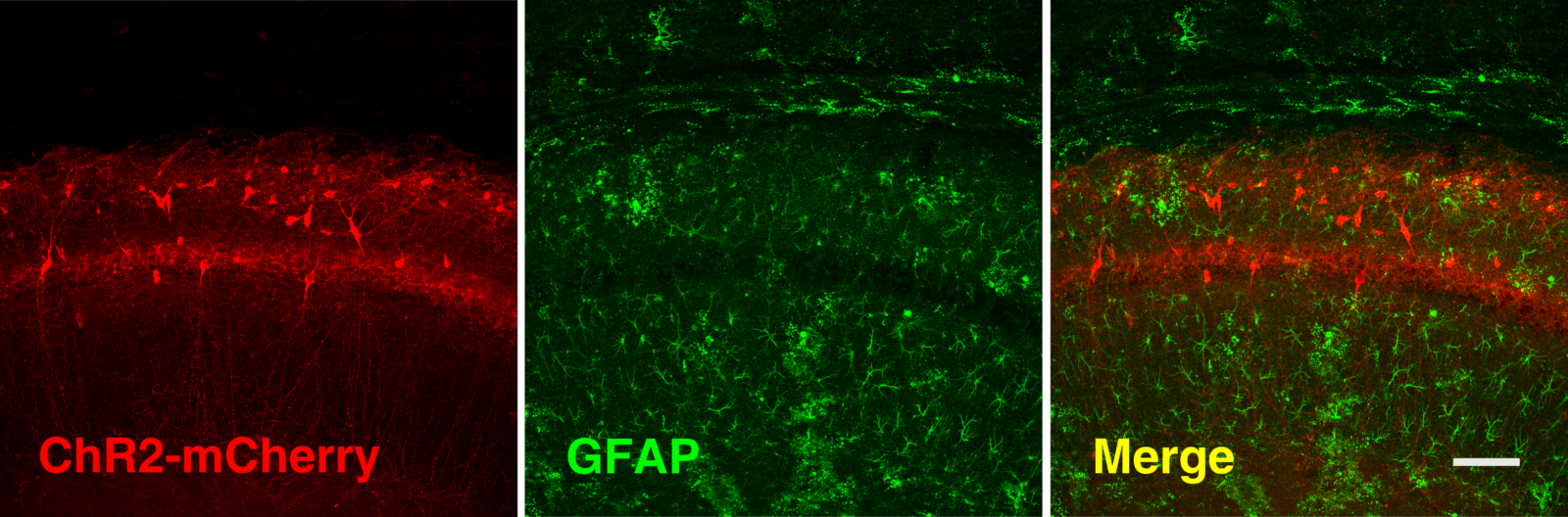
AAV5-GFAP-hM3Dq-mCherry and AAV5-gfaABC1D-cyto-GCaMP6f expression in astrocytes in the CA1 region of the hippocampus. Scale bar, 50 μm.
Team
Eduardo Martín Montiel: Principal Investigator
Samuel Alberquilla Martínez: Postdoctoral researcher
Sara Expósito Reguero: Predoctoral researcher
Pablo Azón Espinar: Predoctoral researcher
Lucía García Carracedo: Research assistant, Comunidad Autónoma de Madrid
Alejandro Hernández Seco: Master Studen. Program JAE Intro, CSIC
Publications
Featured publications
-
Mellado S, Moreno-Ruiz B, Expósito S, Fernández M, Martín ED*. Prolactin Reduces Hippocampal Parvalbumin and GABAA Receptor Expression in Female Mice. Neuroendocrinology. 2021. doi: 10.1159/000520279.
-
Zamora-Moratalla A, Martín ED*. Prolactin enhances hippocampal synaptic plasticity in female mice of reproductive age. Hippocampus. 2021. 31(3):281-293. doi: 10.1002/hipo.23288.
-
Moreno-Ruiz B, Mellado S, Zamora-Moratalla A, Albarracín AL, Martín ED*. Increase in serum prolactin levels in females improves the performance of spatial learning by promoting changes in the circuital dynamics of the hippocampus. Psychoneuroendocrinology. 2021. 124:105048. DOI: 10.1016/j.psyneuen.2020.105048.
-
Lines J, Martín ED, Kofuji P, Aguilar J, Araque A. Astrocytes modulate sensory-evoked neuronal network activity. Nat Commun. 2020. 11:3689. DOI: 10.1038/s41467-020-17536-3.
-
Alberquilla S, Gonzalez-Granillo A, Martín ED**, Moratalla R**. Dopamine regulates spine density in striatal projection neurons in a concentration-dependent manner. Neurobiology of Disease. 2020. 134:104666. DOI: 10.1016/j.nbd.2019.104666.
-
Corkrum M, Covelo A, Lines J, Bellocchio L, Pisansky M, Loke K, Quintana R, Rothwell PE, Lujan R, Marsicano G, Martin ED, Thomas MJ, Kofuji P, Araque A (2020) Dopamine-Evoked Synaptic Regulation in the Nucleus Accumbens Requires Astrocyte Activity. Neuron. 18;105(6):1036-1047.e5. doi: 10.1016/j.neuron.2019.12.026.
-
Caffeine-mediated BDNF release regulates long-term synaptic plasticity through activation of IRS2 signaling. Lao-Peregrín C, Ballesteros JJ, Fernández M, Zamora-Moratalla A, Saavedra A, Gómez Lázaro M, Pérez-Navarro E, Burks D, Martín ED*. Addict Biol. 2017; 22:1706-1718. doi: 10.1111/adb.12433.
-
Synapse-specific astrocyte gating of amygdala-related behavior. Martin-Fernandez M, Jamison S, Robin LM, Zhao Z, Martin ED, Aguilar J, Benneyworth MA, Marsicano G, Araque A. Nat Neurosci. 2017 20(11):1540-1548. doi: 10.1038/nn.4649.
-
The insulin-like growth factor I receptor regulates glucose transport by astrocytes. Hernandez-Garzón E, Fernandez AM, Perez-Alvarez A, Genis L, Bascuñana P, Fernandez de la Rosa R, Delgado M, Angel Pozo M, Moreno E, McCormick PJ, Santi A, Trueba-Saiz A, Garcia-Caceres C, Tschöp MH, Araque A, Martin ED, Torres Aleman I. Glia. 2016; 64:1962-71. doi: 10.1002/glia.23035.
-
Structural and functional plasticity of astrocyte processes and dendritic spine interactions. Perez-Alvarez A, Navarrete M, Covelo A, Martin ED, Araque A. J Neurosci. 2014; 34:12738-44. doi: 10.1523/JNEUROSCI.2401-14.2014.
-
Dopamine release regulation by astrocytes during cerebral ischemia. Oliva I, Fernández M, Martín ED. Neurobiol Dis. 2013; 58:231-41. doi: 10.1016/j.nbd.2013.06.007.
-
Confocal microscopy for astrocyte in vivo imaging: Recycle and reuse in microscopy. Pérez-Alvarez A, Araque A, Martín ED. Front Cell Neurosci. 2013; 7:51. doi:10.3389/fncel.2013.00051.
-
Astrocytes mediate in vivo cholinergic-induced synaptic plasticity. Navarrete M, Perea G, Fernandez de Sevilla D, Gómez-Gonzalo M, Núñez A, Martín ED**, Araque A**. PLoS Biol. 2012; 10:e1001259. doi: 10.1371/journal.pbio.1001259.
-
IRS-2 Deficiency impairs NMDA receptor-dependent long-term potentiation. Martín ED, Sánchez-Perez A, Trejo JL, Martin-Aldana JA, Cano Jaimez M, Pons S, Acosta Umanzor C, Menes L, White MF, Burks DJ. Cereb Cortex. 2012; 22:1717-27. doi: 10.1093/cercor/bhr216.
-
Overexpression of Reelin prevents the manifestation of behavioral phenotypes related to schizophrenia and bipolar disorder. Teixeira CM, Martín ED, Sahún I, Masachs N, Pujadas L, Corvelo A, Bosch C, Rossi D, Martinez A, Maldonado R, Dierssen M, Soriano E. Neuropsychopharmacology. 2011; 36:2395-405. doi: 10.1038/npp.2011.153.
-
Flufenamic acid suppresses epileptiform activity in hippocampus by reducing excitatory synaptic transmission and neuronal excitability. Fernández M, Lao-Peregrín C, Martín ED. Epilepsia. 2010; 51:384-90. doi: 10.1111/j.1528-1167.2009.02279.x.
-
Adenosine released by astrocytes contributes to hypoxia-induced modulation of synaptic transmission. Martín ED, Fernández M, Perea G, Pascual O, Haydon PG, Araque A, Ceña V. Glia. 2007; 55:36-45
Contact
Where to find us
Laboratory of neurophysiology and synaptic plasticity
Instituto Cajal CSIC. Avda. Doctor Arce, 37. 28002. Madrid
Call us
Phone:
Write us
Email address:
Others
Proyects
-
Regulation of neuronal intrinsic properties by gliotransmission. Funding agency: Ministerio de Ciencia e Innovación. Ref: PID2020-116327GB-I00. Dates: 2021/09/01 – 2024/08/31. Principal Investigator: Eduardo D. Martín Montiel.
-
Doctoral Fellow. Subprograma de Formación Personal Investigador (FPI). Project: NMDA-Independent Synaptic Plasticity in Hippocampus. Funding agency: Ministerio de Ciencia e Innovación. Ref: PID2020-116327GB-I00. Dates: 2022/09/01 – 2026/08/31 Fellow: Pablo Azón Espinar. Principal Investigator: Eduardo D. Martín Montiel
-
Synaptic and cellular mechanisms that underlie the functional adaptations of the nervous system during lactation. Funding agency: Ministerio de Ciencia, Innovación y Universidades. Ref: BFU2017-88393-P. Dates: 2018/01/01 – 2020/12/31. Principal Investigator: Eduardo D. Martín Montiel.
-
Simultaneous monitoring of astrocyte and neuronal activity with Electrophysiological and Fiber Photometry techniques in freely-moving animals. Department of Neuroscience, University of Minnesota, USA. Funding agency: Ministerio de Educación y Formación Profesional. “Estancias de movilidad programa Salvador Madariaga”. Ref: PRX19/00646. Principal Investigator: Eduardo D. Martín Montiel.
-
Regulation of synaptic plasticity in the hippocampus by endocrine system. Role of prolactin. Funding agency: Secretaría de Estado de Investigación, Desarrollo e Innovación, MINECO. Ref: BFU2014-57929-P. Dates: 2015/01/01 – 2017/12/31. Principal Investigator: Eduardo D. Martín Montiel.
-
NMDA-Independent Synaptic Plasticity in Hippocampus. Funding agency: Secretaría de Estado de Investigación, Desarrollo e Innovación, Ministerio de Economía y Competitividad. Ref: BFU2011-26339. Dates: 2012/01/01 – 2014/12/31. Principal Investigator: Eduardo D. Martín Montiel.
-
Doctoral Fellow. Subprograma de Formación Personal Investigador (FPI). Project: NMDA-Independent Synaptic Plasticity in Hippocampus. Funding agency: Secretaría de Estado de Investigación, Desarrollo e Innovación, Ministerio de Economía y Competitividad. Ref: BES-2012-052076. Dates: 2013/01/01 – 2016/12/31. Fellow: Alfonsa Zamora Moratalla. Principal Investigator: Eduardo D. Martín Montiel.
-
Grant Cesar Milstein. Funding agency: Ministerio de Ciencia, Tecnología e Innovación Productiva, MINCyT, Argentina. Ref: 568/12. Dates: 2012. Principal Investigator: Eduardo D. Martín Montiel.
-
Synaptic plasticity mechanisms in Parkinson’s disease. Funding agency: Subprograma Juan de la Cierva, Ministerio de Ciencia e Innovación, Subdirección General de Formación e Incorporación de Investigadores. Ref: JCI-2010-07544. Dates: 2011- 2012. Researcher: María Gómez Lázaro. Principal Investigator: Eduardo D. Martín Montiel.
Doctoral Thesis
- Cristina Lao Peregrín. Title: “Synaptic plasticity modulation in the hypocampus mediates by the Insulin Receptor Substrate Proteins”. September 21, 2012. Magna Cum Laude. Universidad de Castilla-La Mancha.
- Idaira Oliva Padrón. Title: “Dopaminergic Synaptic Transmission in the striatum during cerebral ischemia. Regulation by astrocytes”. October 5, 2012. Magna Cum Laude. Universidad de Castilla-La Mancha.
- Jesús Javier Ballesteros. “The role of the BDNF-TrkB system in the NMDA-independent synaptic plasticity induced by xanthines”. September 17, 2015. Magna Cum Laude. Universidad Autónoma de Madrid.
- Alfonsa Zamora Moratalla. “Regulation of synaptic plasticity in the hippocampus by endocrine system. Role of prolactin”. September 8, 2017. Magna Cum Laude. Universidad Autónoma de Madrid. Beca-contrato FPI Ref: BES-2012-052076. Dates: 2013/01/01 – 2016/12/31. Grant: Secretaría de Estado de Investigación, Desarrollo e Innovación, Ministerio de Economía y Competitividad. Ref: BFU2011-26339.
- Susana Mellado Valero. “Morphological-functional correlation of Prolactin-induced changes in synaptic plasticity”. June 7, 2019. Magna Cum Laude. Universidad de Castilla-La Mancha.
- Beatriz Moreno Ruiz. “Role of prolactin in the circuital dynamics of the hippocampus during the learning and spatial memory process.” July 5, 2019. Magna Cum Laude. Universidad de Castilla-La Mancha.
- Samuel Alberquilla Martínez. “Dopaminergic regulation of structural and synaptic plasticity of striatal projection neurons”. December 16, 2022. Co-supervised with Prof. Rosario Moratalla (I. Cajal). Magna Cum Laude, Universidad Autónoma de Madrid.
- Nira Hernández Martín. “Astrocites metabolic changes during temporal lobe epileptogenesis”. Actually on going. Co-supervised with Prof. Miguel Angel Pozo (UCM).
- Sara Expósito Regero. “Astrocitic regulation of the slow Ca2+-activated K+ current. Implication for controlling neuronal excitability”. Actually on going.
- Pablo Azón Espinar. “Bidirectional astrocyte-neuron communication in the motor cortex. Implications in motor neuron disease”. Actually on going

Neuroscience Research Center dependent on the CSIC. Founded in 1920 and initially directed by Santiago Ramón y Cajal. World reference in the study of the brain. Custodian of the Cajal Legacy.
Activities
