LABORATORIES
Astrocyte-neuron interactions in brain circuits and behavioral control
Astrocyte-neuron interactions in brain circuits and behavioral control
Research
Team
Publications
Resources
Contact
Others
Research
Learning and executing everyday tasks, such as walking, drinking, or playing the saxophone, requires our brain to form and reshape its connections. Even the slightest mistake in this process can cause devastating consequences for controlling such basic voluntary behaviors. Indeed, genetic mutations, brain degeneration, stroke, and inflammation cause the insurgence of cognitive and motor diseases, such as Parkinson’s and Alzheimer’s diseases, Obsessive Compulsive Disorder, and frontotemporal dementia. Hence, it is vital to understand these circuits’ function and structure, as well as the underlying cellular, molecular, and genetic mechanisms. In this context, our lab investigates the mechanisms used by astrocytes, the brain’s most abundant glial cell type, and neurons to shape brain circuits and control goal-directed behaviors. First, we aim to describe the causal relationship between structure, function, and proper regulation of goal-directed behaviors.
Moreover, we study the molecular mechanisms by which astrocytes regulate neuronal activity and behavioral output.
Finally, we will unveil the genetic and epigenetic changes induced by learning and adaptation of goal-directed actions that prime astrocytes’ structural and functional modifications.

Lines of investigation
Objective 1. How do astrocytes’ structure and function change upon learning and adaptation of goal-directed behaviors?
We investigate the circular relationship between circuit-specific astrocytes’ structural and functional changes and adult synaptic plasticity.
We use custom-made behavioral setups, machine learning software for video tracking and quantitative, hypothesis-driven behavioral studies, immunohistochemistry, and advanced image quantifications.
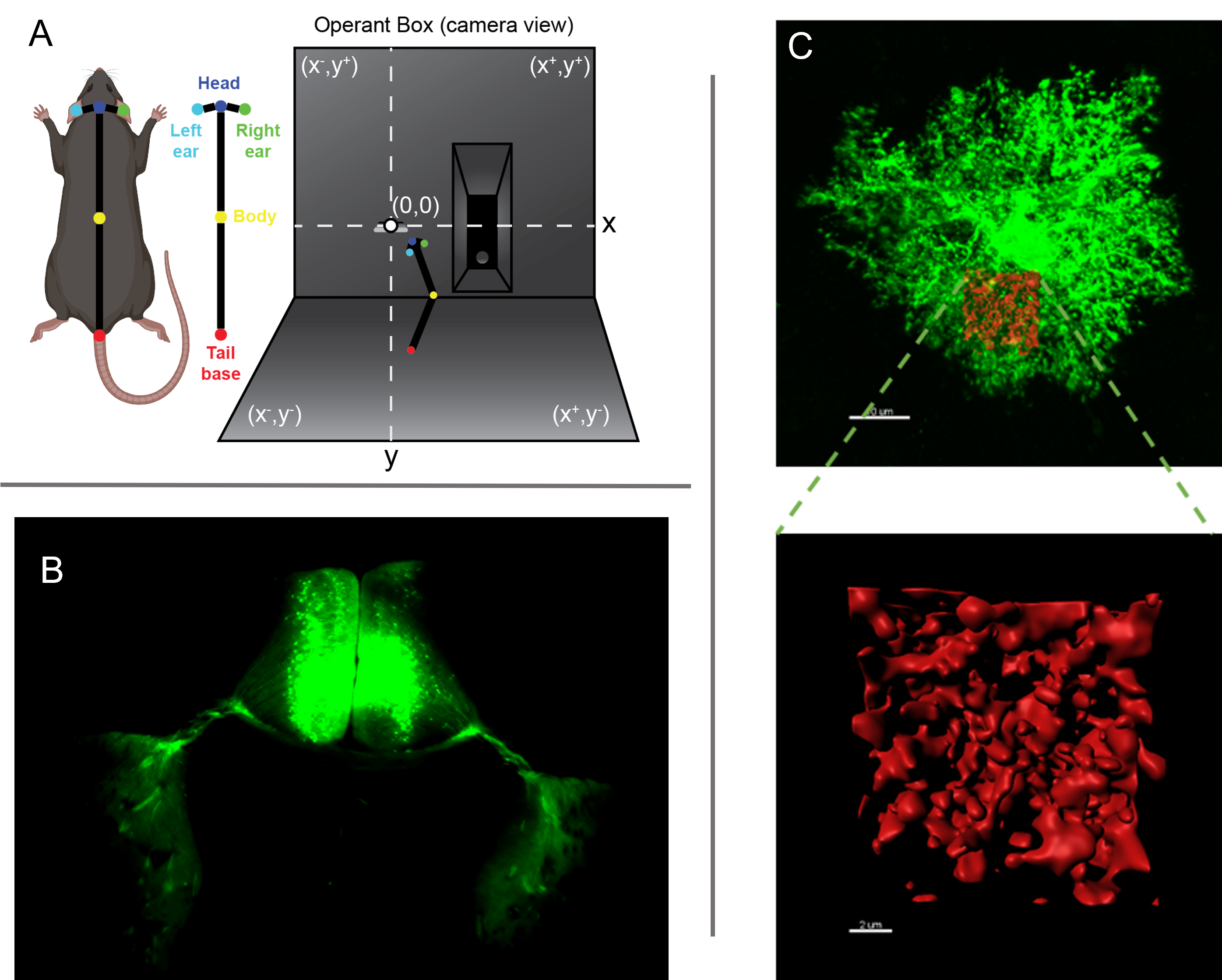
Behavioral video tracking, circuit-specific AAV tracing, and 3D structural analysis of astrocytes morphology as example of some of the approaches used to study astrocytes` role in circuit function and behavioral control.
Objective 2. What molecular pathways are responsible for experience-dependent astrocyte structural and functional modifications?
We investigate the molecular pathways underlying experience-dependent astrocyte structural and functional modifications and their role in health and diseases.
We use in vitro and in vivo models to investigate the intracellular signaling pathways through astrocyte-specific transgenic approaches, optogenetics, chemogenetics, and live-imaging techniques.
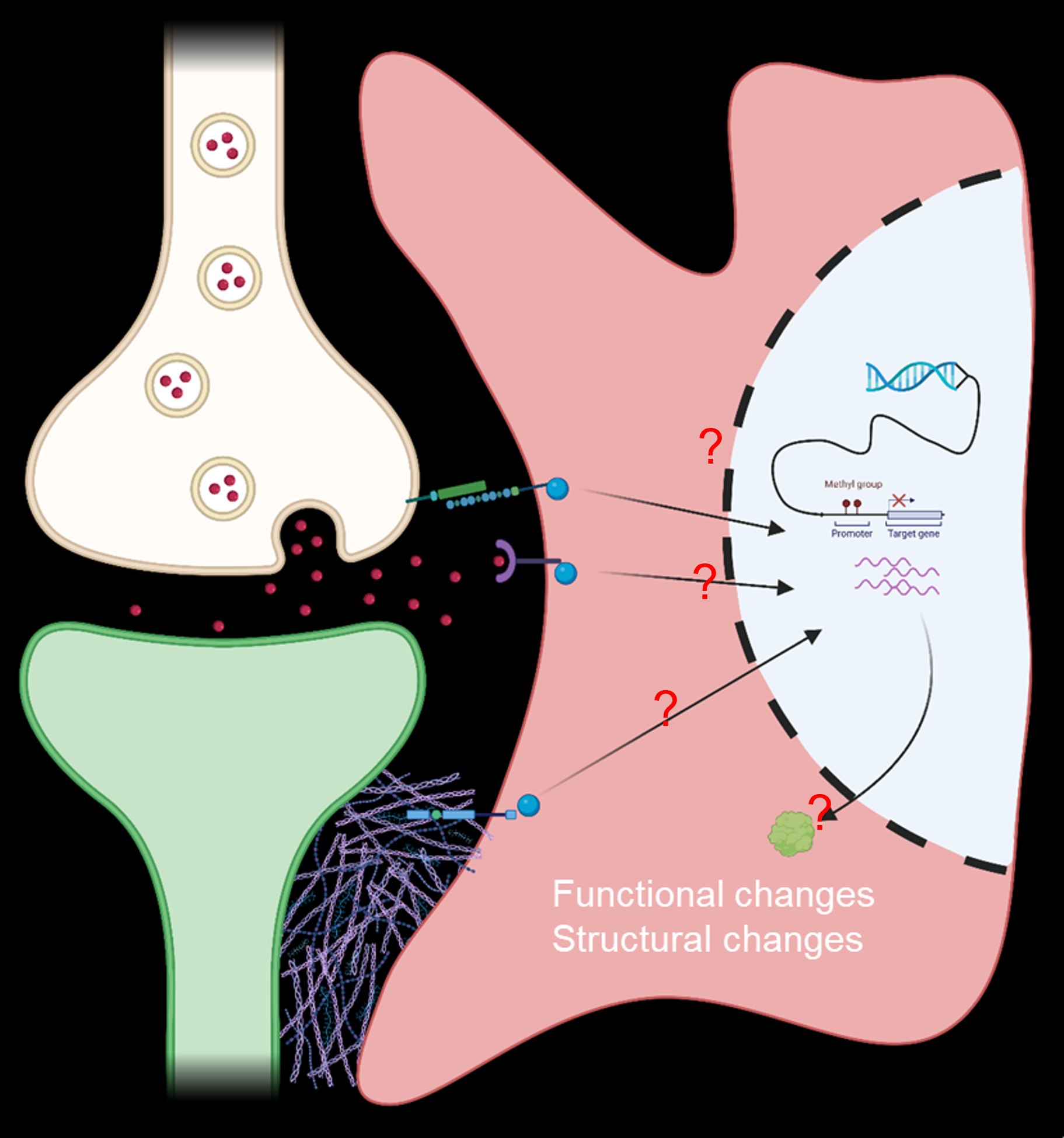
A cartoon of possible unknown intracellular pathways driving structural and functional astrocytic changes. (image created using Biorender.com)
Objetive 3. Astrocytic genetic and epigenetic mechanisms involved in goal-directed actions’ control.
Learning new behaviors and adapting them to changing contingencies leave a “scar” at the genomic level. Understanding how such a process occurs and where could provide novel therapeutic targets and early onset markers to treat or identify brain diseases.
Using transcriptomic and genomic techniques together with cell specific CRISPR/Cas9 methods we aim to unveil novel mechanisms that prime astrocytes for long-term storage of behaviorally relevant information.
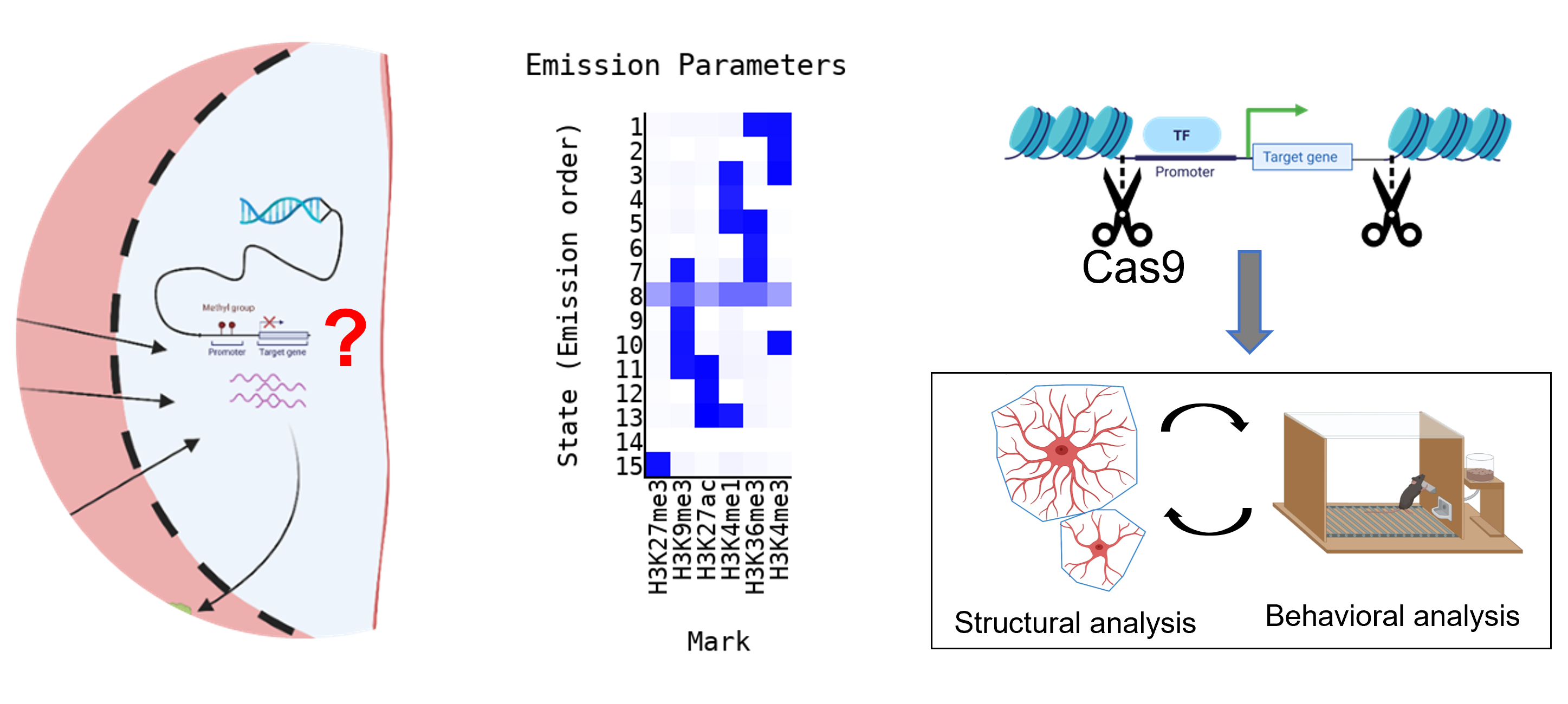
Epigenetic modifications can affect chromatin states and in turn gene expression, astrocytes` structure and function and finally goal-directed behaviors. (image created using Biorender.com)
Team

Francesco Paolo Ulloa Severino
Principal Investigator
Publications
Publications of the last years
-
- Irala D, Wang S, Sakers K, Nagendren L, Ulloa-Severino FP, Sivadasan Bindu D, Savage J. T., Eroglu C. Astrocyte-Secreted Neurocan Controls Inhibitory Synapse Formation and Function. Neuron, 2024. Online resource.
- Ulloa Severino FP*, Lawal O*, Sakers K, Wang S., Kim N, Friedman A, Johnson S., Sriworarat C, Hughes RN, Soderling S, Kim IH, Yin HH, Eroglu C. Training-Induced Circuit-Specific Excitatory Synaptogenesis in mice is Required for Effort Control. Nature Communication, 2023. Online resource.
- Petter EA, Fallon IP, Hughes RH, Watson GDR, Meck WH, Ulloa Severino FP, Yin HH. Elucidating a locus coeruleus-dentate gyrus dopamine pathway for operant reinforcement. eLife, 2023. Online resource.
- Lawal O, Ulloa Severino FP*, Eroglu C*. The role of astrocyte structural plasticity in regulating neural circuit function and behavior. Glia, 2022. Online resource.
- Zhang J, Hughes RN, Kim N, Fallon IP, Bakhurin K, Kim K, Ulloa Severino FP, Yin HH A one-photon endoscope for simultaneous patterned optogenetic stimulation and calcium imaging in freely behaving mice. Nature Biomedical Engineering, 2022. Online resource.
- Watson GDR, Hughes RN, Petter EA, Fallon IP, Kim N, Ulloa Severino FP, Yin HH. Thalamic projections to the subthalamic nucleus contribute to movement initiation and rescue of parkinsonian symptoms. Science Advances, 2021. Online resource.
- Cortés-Llanos B, Ulloa Severino FP. 3D Carbon-based scaffolds for brain model and tissue engineering. STEMedicine, 2020. Online resource.
- Xiao M, Ulloa Severino FP, Iseppon F, Cheng G, Torre V, Tang M. 3D Free-Standing Ordered Graphene Network Geometrically Regulates Neuronal Growth and Network Formation. Nano Letters, 2020. Online resource.
- Dedola F*, Ulloa Severino FP*, Meneghetti N, Lemaire T, Cafarelli A, Ricotti L, Menciassi A, Cutrone A, Mazzoni A, Micera S. Ultrasound Stimulations Induce Prolonged Depolarization and Fast Action Potentials in Leech Neurons. Engineering in Medicine and Biology, 2020. Online resource.
- Tigani W, Pinzan Rossi M, Artimagnella O, Santo M, Rauti R, Sorbo T, Ulloa Severino FP, Provenzano G, Allegra M, Caleo M, Ballerini L, Bozzi Y, Mallamaci A. Foxg1 Upregulation Enhances Neocortical Activity. Cerebral cortex, 2020. Online resource.
- Rauti R, Secomandi N, Martín C, Bosi S, Ulloa Severino FP, Scaini D, Prato M, Vázquez E, Ballerini L. Tuning Neuronal Circuit Formation in 3D Polymeric Scaffolds by Introducing Graphene at the Bio/Material Interface. Advanced Biosystem, 2020. Online resource.
- Xiao X, Li X, Song Q, Zhang Q, Lazzarino M, Cheng G*, Ulloa Severino FP*, Torre V*. A Fully 3D Interconnected Graphene–Carbon Nanotube Web Allows the Study of Glioma Infiltration in Bioengineered 3D Cortex-Like Networks. Advanced Materials, 2018. Online resource.
*These authors contributed equally. For the complete publication list, please access the ORCID website
Resources
UlloaSeverinoLab Github: https://github.com/UlloaSeverinoLab
Contact
Where to find us
Ulloa Severino´s laboratory, room C01
Instituto Cajal CSIC. Avda. Doctor Arce, 37. 28002. Madrid
Call us
Phone number:
Write us
Email address:
Others
Granted projects
2023-28: Astrocyte-neuron cellular and structural interactions to control brain circuits and behavior. Financing Agency: Ministro de Ciencia y Innovación, Agencia Estatal de investigación, and NextGenerationEU. Reference Number: RYC2021-033202-I. Total amount: 236.250 €.
2022: Semifinalist Award from the Joe W. and Dorothy Dorsett Brown Foundation. Total amount: 15,000 $.
Supervised Theses
- Investigating a novel intracellular signaling pathway controlling astrocytes’ structural plasticity
Irene Torres Pulido (2023)
Universidad de Alcalá
Trabajo de fin de Grado
Colaborations
Cagla Eroglu, Dep. of Cell Biology. Duke University, Durham, North Carolina (USA).
Henry Yin, Dep. of Psychology and Neuroscience. Duke University, Durham, North Carolina (USA).
Maria Angeles Arévalo, Dep. of Functional and Systems Neurobiology. Instituto Cajal (CSIC).

Neuroscience Research Center dependent on the CSIC. Founded in 1920 and initially directed by Santiago Ramón y Cajal. World reference in the study of the brain. Custodian of the Cajal Legacy.
Activities
