LABORATORIES
Molecular control of neurogenesis
Control molecular de la neurogénesis
Research
Team
Publications
Contact
Others
Research
Our laboratory is interested in understanding the molecular bases that govern the generation of new neurons, both during the development of the nervous system and in the adult brain. The generation of new neurons or neurogenesis is maintained, in a limited way, in discrete niches of the adult nervous system of many vertebrates throughout the life of the animal and contributes to the plasticity of the nervous system. However, this adult neurogenesis is highly restricted, both in total number and in distinct neural subtypes generated. Our lab is interested in exploring how rates of cell generation and the temporal extent of neurogenesis in the adult brain can be modulated in comparison to the neurogenesis process during development.

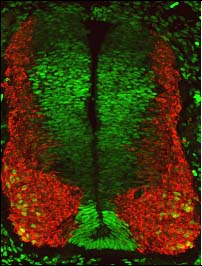
Over the years, we have contributed to understanding the signals and genetic programs that govern neural development. Regarding the signals, we have shown that FGF and retinoic acid signaling are essential for the temporal control of the specification and migration of neural crest cells (Martínez-Morales, et al., 2011, Diez del Corral and Morales, 2014). Furthermore, we have shown that the FGF pathway modulates the activity of the Shh signaling pathway to control the ventral identity of the spinal cord (Morales et al., 2016).
We have also contributed to establishing how neurogenesis and cell fate specification are controlled during development through the modulation of signaling pathways, such as the canonical Wnt pathway. In this sense, the laboratory has also established how intrinsic factors, such as the Sox5 transcription factor, promote cell cycle exit in neural progenitors and the specification of interneuronal subtypes in the developing spinal cord, modulating the canonical Wnt pathway (Martinez -Morales et al., 2010; Quiroga et al., 2015).
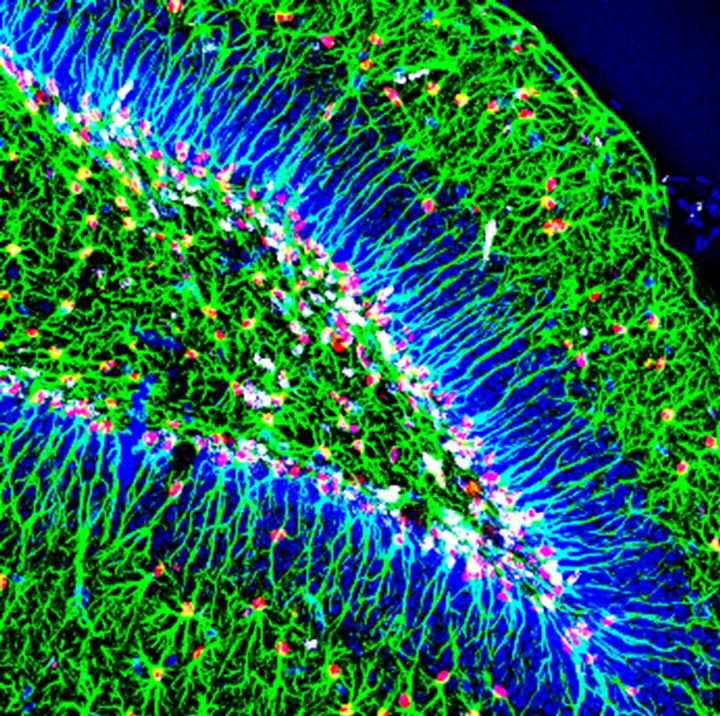
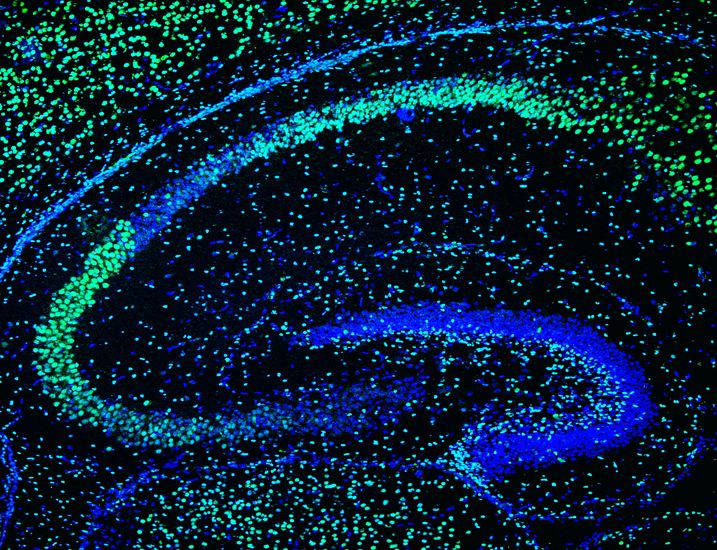
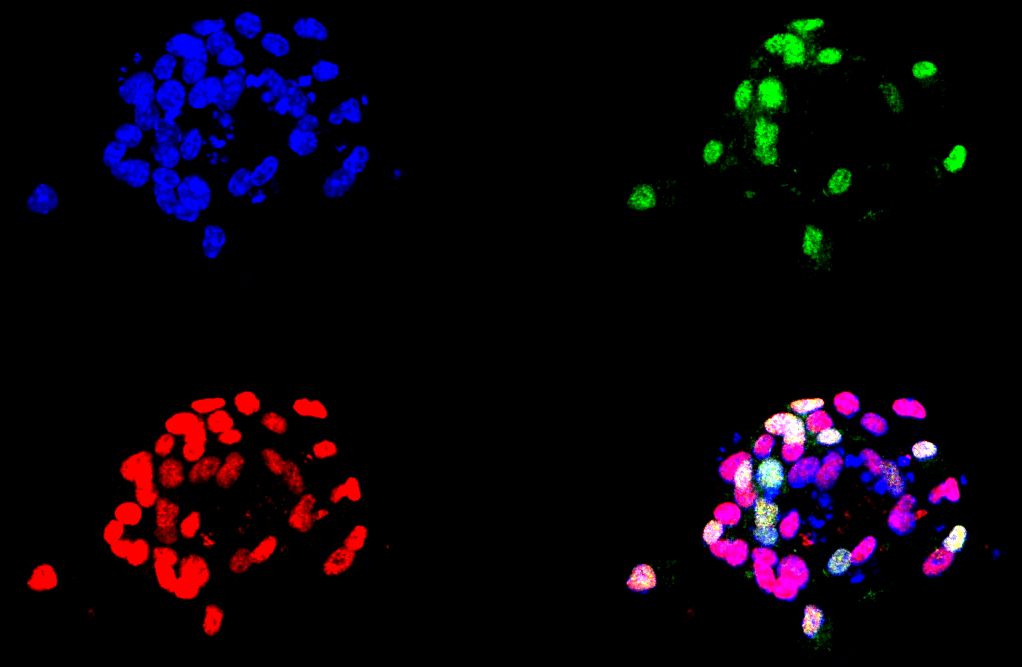
We are currently studying how new neurons are generated from stem cells in the adult brain and when and how, during brain development, adult neural stem cells were generated in certain specific areas of the brain, such as the dentate gyrus ( GD) of the hippocampus (Mira and Morales, 2019). In adult GD, neural stem cells (NSCs) generate new granule cells throughout life. Most of these MNCs are in a reversible state of quiescence, which protects the cells’ DNA from possible damage and prevents depletion of the neural pool. However, little is known about how the acquisition of quiescence is regulated and how the transition from quiescence to an active mitotic state is controlled. To understand this key process, our laboratory is exploring the consequences of conditional lack of Sox5 and Sox6 function in the adult mouse hippocampus and during dentate gyrus development, using both in vivo and in vitro (neurosphere culture) approaches.
In addition, we are also interested in the potential of adult neural stem cells as therapeutic tools in neurodegenerative diseases. We have recently discovered how inhibitors of a protein involved in neurodegenerative diseases (LRRK2) can promote the generation of oligodendrocytes and neurons from neuronal stem cells of the adult brain (Zaldívar et al., 2020).
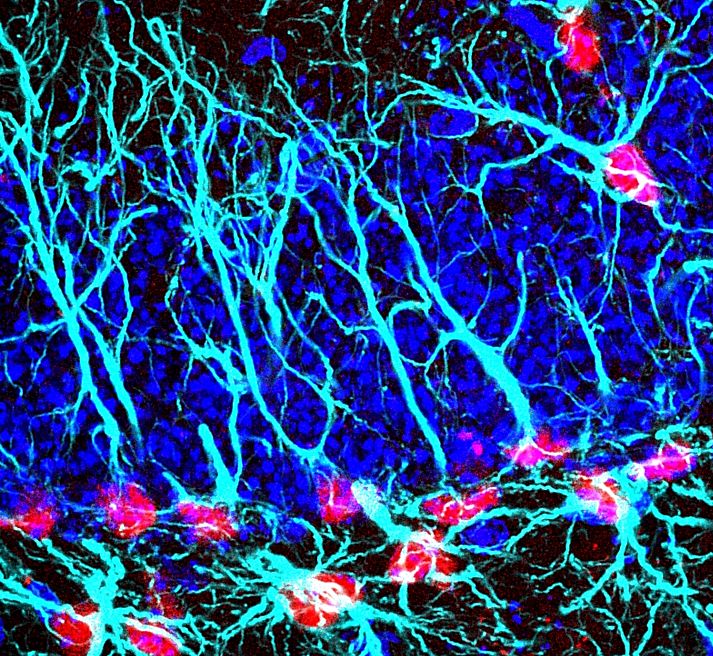
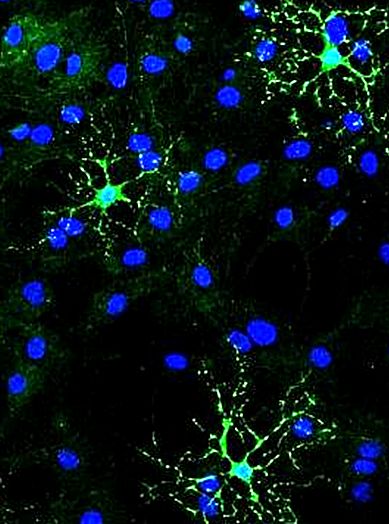
Team

Aixa Morales
Principal Investigator
Publications
Recent publications
- Sublayer- and cell-type-specific neurodegenerative transcriptional trajectories in hippocampal sclerosis.(2021). E. Cid, A. Márquez-Galera, M. Valero, B. Gal, D. C. Medeiros, C. M. Navarrón, L. Ballesteros-Esteban, R. Reig-Viader, Morales, A.V. L. Dolon, I. Fernandez-Lamo, D. Gomez-Dominguez, M. Sato, Y. Hayashi, À. Bayés, A. Barco, J. P López-Atalaya, L. M de la Prida.Cell Reports 35(10):109229
- Editorial: Generation of Neurons and Their Integration in Pre-existing Circuits in the Postnatal Brain: Signalling in Physiological and Regenerative Contexts. (2020). Mira H, Diez Del Corral R, Morales, A.V.Front Cell Dev Biol. 8:560 (2020) Electronic Book Collection 2020.
- Benzothiazole-based LRRK2 inhibitors as WNT enhancers and promoters of oligodendrocytic fate. (2020). Zaldivar-Diez, J., Li, L., García, A.M., Zhao , W., Medina-Menendez, C., Haggarty, S.J., Gil, C., Morales, A.V.* and Martinez, A*. (*corresponding authors).Dec 26. doi: 10.1021/acs.jmedchem.9b01752). [Epub ahead of print]
- Adult neural stem cells: born to last. (2019). A. V. Morales, and H. MiraFront. Cell Dev. Biol. Jun 4;7: 96
- Proximodistal organization of the CA2 hippocampal area. (2019). I. Fernandez-Lamo, D. Gomez-Dominguez, A. Sanchez-Aguilera, A. Oliva, A. V. Morales, M. Valero, E. Cid, A. Berenyi and L. Menendez de la Prida.Cell Reports 26 (7): 1734-1746 [Cover]
- A focused library of psychotropic analogs with neuroprotective and neuroregenerative potential. (2019). Uliassi, E.; Peña-Altamira, L. E.; Morales, A.V.; Massenzio, F.; Petralla, S; Rossi, M; Roberti, M.; Martinez Gonzalez, L.; Martínez, A.; Monti, B.; Bolognesi, M. L. ACS Chemical Neuroscience Jan 16;10(1):279-294.
- GLI1 Inactivation is associated with Developmental Phenotypes Overlapping with Ellis-Van Creveld Syndrome (2017).A. Palencia-Campos, A. Ullah, J.Nevado, R. Yıldırım, E.Unal, M. Ciorraga, P., Lucia Chico, F. Piceci-Sparascio, V. Guida, A. De Luca, H. Kayserili, I. Ullah, M. Burmeister, P. Lapunzina, W. Ahmad, Morales, A.V., V. L. Ruiz-Perez. Human Molecular Genetics. 26(23):4556-4571. [Cover]
- Leucine Rich Repeat Kinase 2 (LRRK2) Inhibitors based on indolinone scaffold: Potential Pro-neurogenic Agents. (2017). Salado, I.G., Zaldivar-Diez, J., Sebastian, V., Li, L., Geiger, L., González, S., Campillo, N.E., Morales, A.V.,, Perez, D.I. and Martinez, A. European Journal of Medicinal Chemistry 138:328-342.
- The multiple roles of FGF signaling in the extending spinal cord. (2017). Diez del Corral, R. and Morales, A.V. Front. Cell Dev. Biol. Jun 2;5:58.
- Brain insulin-like growth factor-I directs the transition from stem cells to mature neurons during postnatal/adult hippocampal neurogenesis. (2016). Nieto-Estevez, V, Oueslati-Morales, C.O., Li, L., Pickel, J., Morales, A.V., and Vicario-Abejon. Stem Cells 34(8):2194-209.
- FGF signaling enhances a Shh negative feedback loop to coordinate ventral patterning and caudal extension of the spinal cord.(2016). Morales, A.V., Espeso-Gil, S., Ocaña, I., Nieto-López, F., Calleja, E., Bovolenta, P., Lewandoski, M. and Diez del Corral, R.Developmental Neurobiology 76(9):956-71.
- Neural development and regeneration: it’s all in your spinal cord.(2015). Becker CG, Diez Del Corral R. Development 142: 811-816.
- Sox5 controls dorsal progenitor and interneuron specification in the spinal cord.(2014).Quiroga AC, Stolt CC, Diez del Corral R, Dimitrov S, Perez-Alcala S, Sock E, Barbas JA, Wegner M, Morales AV.Developmental Neurobiology. 75(5):522-38 (2015)
- Retinoic Acid Signaling during Early Spinal Cord Development.(2014).Morales AV, Diez del Corral R. J Dev Biol 2: 174-197.
- FGF and retinoic acid activity gradients control the timing of neural crest cell emigration in the trunk. (2011). Martinez-Morales, P.L., Diez Del Corral, R., Olivera-Martinez, I., Quiroga, A.C., Das, R.M., Barbas, J.A., Storey, K.G., and Morales, A.V.J Cell Biol. 194:489-503.
- Coordination of Cell Differentiation and Migration in Mathematical Models of Caudal Embryonic Axis Extension. (2011). Harrison, N.C., Diez del Corral, R.*, and Vasiev, B.*PLoS ONE 6, e22700.*Co-corresponding authors.
- SOX5 controls cell cycle progression in neural progenitors by interfering with the WNT-beta-catenin pathway.(2010). Martinez-Morales, P.L., Quiroga, A.C., Barbas, J.A., and Morales, A.V.EMBO Rep 11, 466-472.
- Snail genes at the crossroads of symmetric and asymmetric processes in the developing mesoderm(2007). Morales AV, Acloque H, Ocaña OH, de Frutos CA, Gold V and Nieto MA. EMBO Reports. 8:104-109 [cover caption].
- How to become neural crest: from segregation to delamination(2005) Morales AV, Barbas JA, Nieto MA.Semin Cell Dev Biol. 16(6):655-62.
- LSox5 regulates RhoB expression in the neural tube and promotes generation of the neural crest(2004). Perez-Alcala S, Nieto MA, Barbas JA. Development 131(18):4455-65.
Related lab member publications
- Snail blocks the cell cycle and confers resistance to cell death.(2004). Vega, S., Morales, A.V., Ocana, O.H., Valdes, F., Fabregat, I., and Nieto, M.A.Genes Dev 18, 1131-1143.
- The snail gene family in gastrulation.(2004). Morales, A.V. and Nieto M.A.In: Gastrulation. Ed: Stern, C. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York.
- Opposing FGF and retinoid pathways: a signalling switch that controls differentiation and patterning onset in the extending vertebrate body axis.(2004). Diez del Corral, R., and Storey, K.G.Bioessays 26, 857-869.
- Opposing FGF and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension(2003).Diez del Corral, R., Olivera-Martinez, I., Goriely, A., Gale, E., Maden, M. and Storey, K.Neuron 40, 65-79.
- Periodic Lunatic fringe expression is controlled during segmentation by a cyclic transcriptional enhancer responsive to notch signalling (2002). Morales AV, Yasuda Y, Ish-Horowicz D.Dev Cell. 3(1):63-74.[ highlights in Current Biology 12 :R699-R701 (2002); The Scientist 16 (20) :45 (2002) and in Faculty of 1000, 2002.
Contact
Where to find us
Molecular control of neurogenesis laboratory
Instituto Cajal CSIC. Avda. Doctor Arce, 37. 28002. Madrid
Call us
Phone:
Write us:
Email address:
Others
Doctoral Thesis
- Ling Li: “Role of Sox5 and Sox6 in adult hippocampal neurogenesis”. Facultad de Ciencias, Universidad Autónoma de Madrid. (18/12/2020; Sobresaliente cum laude por unanimidad).
- Alejandra Quiroga del Río: “Función de Sox5 y Sox6 en el desarrollo del sistema nervioso de pez medaka”.Facultad de Ciencias, Universidad Autónoma de Madrid. (22/03/2013; Sobresaliente cum laude por unanimidad; Mención europea).
- Patricia Martínez Morales: “Bases moleculares del desarrollo de la cresta neural troncal”. Facultad de Ciencias, Universidad Autónoma de Madrid (25 de Febrero del 2011, Sobresaliente cum laude por unanimidad; Premio Extraordinario de Doctorado, Dept. Bioquímica, UAM).
Collaborations
- Liset Menéndez de la Prida (Instituto Cajal)
- José Luís Trejo (Instituto Cajal)
- Helena Mira Instituto de Biomedicina de Valencia, CSIC)
- Carlos Vicario Abejón (Instituto Cajal)
- Ana Martínez (Centro de Investigaciones Biológicas, CSIC)
- Victor Ruíz (Instituto de Investigaciones Biomédicas “Alberto Sols”, CSIC)
- Silvia Nicolis (University Milano Bicoca, Italy).
Funding
- Análisis de la neurogénesis hipocampal durante el desarrollo y la etapa adulta a través del estudio funcional de los genes SoxD. MCINN SAF2017-85717-R (2018-2021)
- Análisis de la participación hipocampal en las interacciones sociales en un modelo genético de trastorno del espectro autista. Fundación Alicia Koplowitz (2018-2020)

Neuroscience Research Center dependent on the CSIC. Founded in 1920 and initially directed by Santiago Ramón y Cajal. World reference in the study of the brain. Custodian of the Cajal Legacy.
Activities
