LABORATORIES
Basal ganglia neurobiology
Basal ganglia neurobiology
Research
Team
Publications
Contact
Others
Research
Our laboratory has extensive experience in the physiology and pathology of the basal ganglia, the neuroanatomical substrate of Parkinson’s Disease (PD) and other neurological disorders such as drug addiction.
We are interested in studying the molecular mechanisms involved in dopaminergic degeneration and dyskinesias induced by L-DOPA. In particular, we study the mechanisms responsible for the structural and synaptic plasticity of striatal projection neurons in PD and the role played by dopaminergic receptors. We are also interested in determining the anatomical and molecular bases involved in the development of non-motor symptoms of PD and mental disorders resulting from L-DOPA treatment and identifying the neuronal circuits involved, as well as their causes. The elucidation of the molecular mechanisms that underlie the pathophysiology of PD is essential to develop therapeutic strategies that can stop or slow down the progression of the disease.
To address these objectives, we use sophisticated methodologies such as:
- Optogenetics and pharmacogenetics
- Fibrometry
- In vivo and in vitro electrophysiology techniques
- Imaging techniques
- Behavior test
- Immunohistochemistry, immunofluorescence and molecular biology techniques.
- Transgenic animal models of PD.
- Patient-specific cellular models of PD.

Lines of investigation
1) Structural and synaptic plasticity of the basal ganglia
Dopaminergic denervation of striatal neurons and loss of axo-spinous synapses that carry motor information from the substantia nigra (SN) are the hallmarks of PD. In the laboratory we study the mechanisms responsible for this synaptic remodeling and how it varies with L-DOPA treatment to produce dyskinesias. We have developed strategies to study this plasticity in the two main types of striatal neurons that control movement and its causal relationship with motor response in PD and dyskinesias.
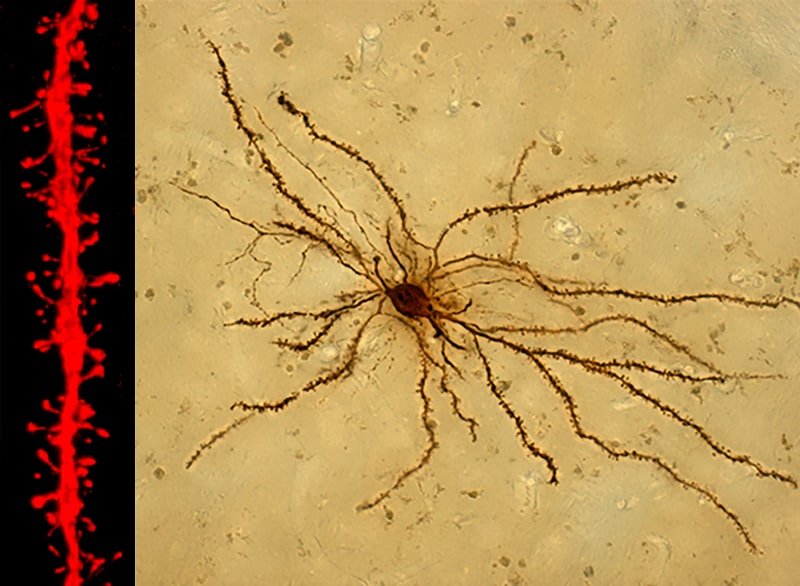
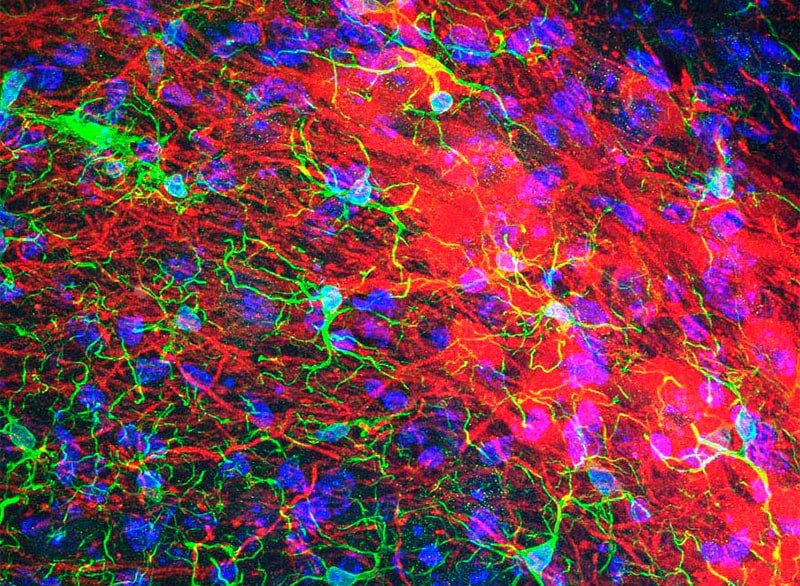
2) Impulse Control Disorder in PD
Although dopamine replacement therapy relieves the motor symptoms of PD, over time, it leads to disabling complications. In addition to dyskinesias, this therapy can induce Impulse Control Disorder, a psychiatric condition that affects more than 13% of patients and is characterized by the inability to curb impulses, giving rise to risky behaviors such as compulsive gambling, shopping compulsive or hypersexual To understand this disorder, we used multidisciplinary approaches combining electrophysiological recordings of neural circuits that process motivational and reward information with optogenetic techniques in transgenic animal models of PD.
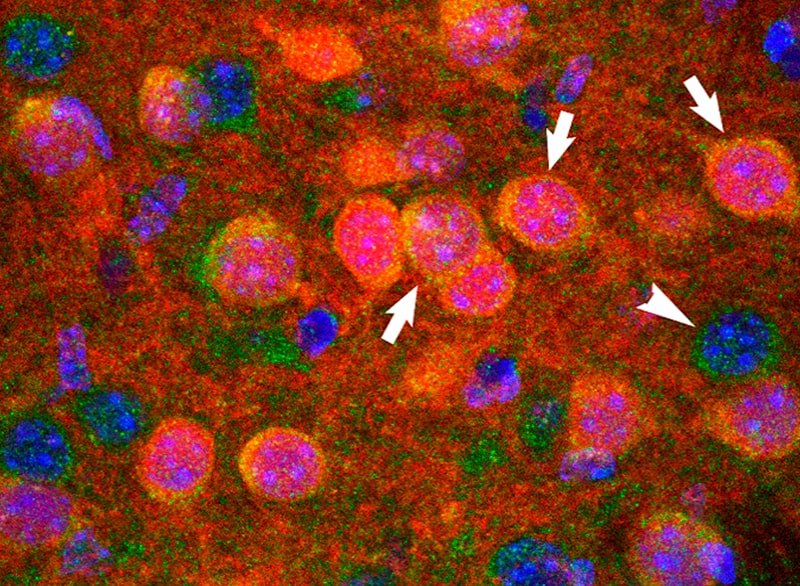
3) Non-motor symptoms of Parkinson’s disease
Non-motor symptoms such as anxiety/depression and gastrointestinal disorders affect up to 50% and 80% of PD patients, respectively. These early alterations aggravate the evolution of the disease, profoundly affecting the quality of life of patients. Despite the fact that its clinical presence in PD was demonstrated several years ago, the underlying basis remains unknown. We used animal models of PD and postmortem brain samples from PD patients to determine the neural circuitry and molecular basis that govern the development of these nonmotor symptoms.
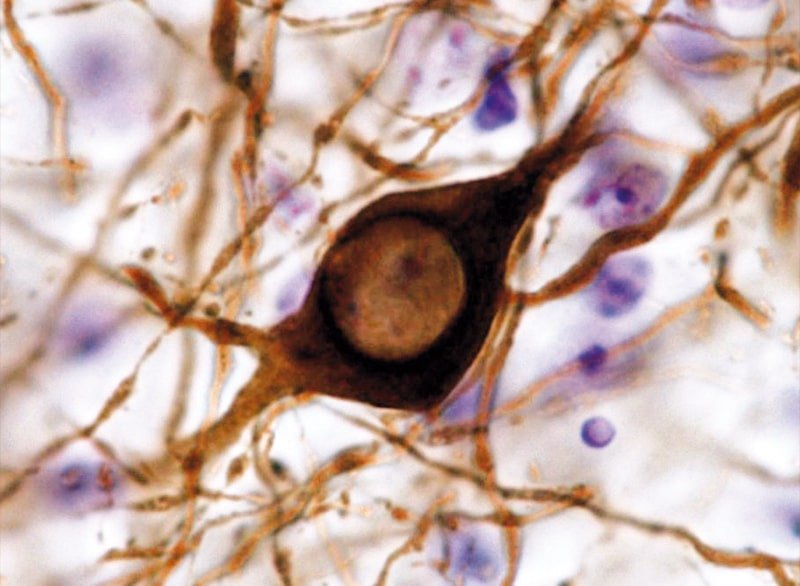
4) Neuropathological bases of PD. therapeutic strategies
The aggregation of the αSyn protein is the main neuropathological feature of PD. However, the mechanisms that determine their aggregation as well as dopaminergic neuronal death remain largely unknown. Mutations in the GBA1 gene are the main genetic risk factor for PD and promote αSyn aggregation. To determine the bases that govern the neuropathology of PD, we used animal models of αSyn and fibroblasts derived from parkinsonian patients carrying GBA1 mutations. In addition, we are evaluating the therapeutic potential of different strategies designed to stop the progression of PD.
Personal

Rosario Moratalla Villalba
Group manager
Research Professor

Noelia Granado Martínez
Postdoctoral researcher

Elena Juárez Escoto
Postdoctoral researcher

Adrián Sanz Magro
Postdoctoral researcher

Juan Enríquez Traba
Predoctoral researcher

Mario García-Verdugo Jiménez-Egizabal
Predoctoral researcher

Mónica Gómez Benito
Predoctoral researcher

Carlos Salas Prieto
Predoctoral researcher

Aida de la Fuente Murillo
Laboratory technician

Sara Murillo Alonso
Laboratory technician
Publications
Most relevant publications
- Selective activation of striatal indirect pathway suppresses levodopa induced-dyskinesias. Castela I, Casado-Polanco R, Rubio YV, da Silva JA, Marquez R, Pro B, Moratalla R, Redgrave P, Costa RM, Obeso J, Hernandez LF. Neurobiol Dis. 2023 Jan;176:105930. doi: 10.1016/j.nbd.2022.105930
- Motor cortico-nigral and cortico-entopeduncular information transmission and its modulation by buspirone in control and after dopaminergic denervation. Vegas-Suárez S, Morera-Herreras T, Requejo C, Lafuente JV, Moratalla R, Miguélez C, Ugedo L. Front Pharmacol. 2022 Aug 30;13:953652. doi: 10.3389/fphar.2022.953652.
- Metabolic Diffusion in Neuropathologies: The Relevance of Brain-Liver Axis. Vegas-Suárez S, Simón J, Martínez-Chantar ML, Moratalla R. Front Physiol. 2022 May 12;13:864263. doi: 10.3389/fphys.2022.864263.
- Restricting feeding to dark phase fails to entrain circadian activity and energy expenditure oscillations in Pitx3-mutant Aphakia mice. Fernández-Pérez A, Sanz-Magro A, Moratalla R, Vallejo M. Cell Rep. 2022 Jan 11;38(2):110241. doi: 10.1016/j.celrep.2021.110241.
- Genetic deletion of dopamine D1 receptors increases the sensitivity to cannabinoid CB1 receptor antagonist-precipitated withdrawal when compared with wild-type littermates: studies in female mice repeatedly exposed to the Spice cannabinoid HU-210. Serrano A, Vadas E, Ferrer B, Bilbao A, Granado N, Suárez J, Pavon FJ, Moratalla R, Rodríguez de Fonseca F. Psychopharmacology (Berl). 2021 Feb;238(2):551-557. doi: 10.1007/s00213-020-05704-8. Epub 2021
- Dopamine DR2R is required for Hippocampal-dependent Memory and Plasticity at the CA2-CA1 Synapse. Espadas I, Ortiz O, García-Sanz P, Sanz-Magro A, Alberquilla S., Solis O, Delgado-García JM, Gruart A & Moratalla R. Cereb Cortex. 2021 Mar 5; 31(4): 2187-2204. doi:10.1093/cercor/bhaa354. PMID:33264389.
- The Role of Cholesterol in α-Synuclein and Lewy Body Pathology in GBA1 Parkinson’s Disease. García-Sanz, P., M F G Aerts, J., & Moratalla, R. Movement Disorders. 2020 Nov. doi: org/10.1002/mds.28396.
- Sex-specific behavioral and neurogenic responses to cocaine in mice lacking and blocking dopamine D1 or dopamine D2 receptors. Rivera, P., Aranda, J., Alén, F., Vargas, A., Serrano, A., Pavón, F. J., Orio, L., Rubio, L., Moratalla, R., de Fonseca, F. R., & Suárez, J. The Journal of comparative neurology. 2021. 529(8):1724-1742. doi: 10.1002/cne.25052.
- Diabetes Causes Dysfunctional Dopamine Neurotransmission Favoring Nigrostriatal Degeneration in Mice. Pérez-Taboada, I., Alberquilla, S., Martín, E. D., Anand, R., Vietti-Michelina, S., Tebeka, N. N., Cantley, J., Cragg, S. J., Moratalla, R., & Vallejo, M. Movement Disorders. 2020 Sep. 35(9):1636-1648. doi: 10.1002/mds.28124.
- Dopamine D1 Receptors Regulate Spines in Striatal Direct-Pathway and Indirect-Pathway Neurons. Suarez, L. M., Solis, O., Sanz-Magro, A., Alberquilla, S., & Moratalla, R. Movement Disorders. 2020 Oct. 35(10):1810-1821. doi: 10.1002/mds.28174.
- Modeling Parkinson disease with the alpha-synuclein protein. Gómez-Benito M, Granado N, García-Sanz P, Michel A, Dumoulin M, and Moratalla R. Front. Pharmacol. 2020 Apr. 11:356. doi: 10.3389/fphar.2020.00356.
- Beneficial effects of the phytocannabinoid Δ9-THCV in L-DOPA-induced dyskinesia in Parkinson’s disease. Espadas, I., Keifman, E., Palomo-Garo, C., Burgaz, S., García, C., Fernández-Ruiz, J., & Moratalla, R. Neurobiology of disease. 2020 Jul. 141:104892. doi: 10.1016/j.nbd.2020.104892.
- DRD3 (dopamine receptor D3) but not DRD2 activates autophagy through MTORC1 inhibition preserving protein synthesis. Barroso-Chinea, P., Luis-Ravelo, D., Fumagallo-Reading, F., Castro-Hernandez, J., Salas-Hernandez, J., Rodriguez-Nuñez, J., Febles-Casquero, A., Cruz-Muros, I., Afonso-Oramas, D., Abreu-Gonzalez, P., Moratalla, R., Millan, M. J., & Gonzalez-Hernandez, T. Autophagy. 2020 Jul. 16(7):1279-1295. doi: 1080/15548627.2019.1668606.
- Dopamine regulates spine density in striatal projection neurons in a concentration-dependent manner. Alberquilla S, Gonzalez-Granillo A, Martín ED, Moratalla R. Neurobiol. Dis. 2020 Feb. 134:104666. doi: 10.1016/j.nbd.2019.104666.
- A collection of three integration-free iPSCs derived from old male and female healthy subjects. Rodríguez-Traver E, Díaz-Guerra E, Rodríguez C, Arenas F, Orera M, Kulisevsky J, Moratalla R, and Vicario C. Stem Cell Res. 2020 Jan. 2:101663. doi: 10.1016/j.scr.2019.101663.
- Behavioral sensitization and cellular responses to psychostimulants are reduced in D2R knockout mice. Solís O, García-Sanz P, Martín AB, Granado N, Sanz-Magro A, Podlesniy P, Trullas R, Murer MG, Maldonado R, and Moratalla R. Addict Biol. 2019 Dec. 12:e12840. doi: 10.1111/adb.12840.
- Hypomorphic Expression of Pitx3 Disrupts Circadian Clocks and Prevents Metabolic Entrainment of Energy Expenditure. Del Río-Martín A, Pérez-Taboada I, Fernández-Pérez A, Moratalla R, de la Villa P, Vallejo M. Cell Rep. 2019 Dec. 29(11):3678-3692.e4. doi: 10.1016/j.celrep.2019.11.027.
- Generation of an integration-free iPSC line, ICCSICi005-A, derived from a Parkinson’s disease patient carrying the L444P mutation in the GBA1 gene. Rodríguez-Traver E, Rodríguez C, Díaz-Guerra E, Arenas F, Araúzo-Bravo M, Orera M, Kulisevsky J, Moratalla R, Vicario C. Stem Cell Res. 2019 Oct. 40:101578. doi: 10.1016/j.scr.2019.101578.
- DRD3 (dopamine receptor D3) but not DRD2 activates autophagy through MTORC1 inhibition preserving protein synthesis. Barroso-Chinea P, Luis-Ravelo D, Fumagallo-Reading F, Castro-Hernandez J, Salas-Hernandez J, Rodriguez-Nuñez J, Febles-Casquero A, Cruz-Muros I, Afonso-Oramas D, Abreu-Gonzalez P, Moratalla R, Millan MJ, Gonzalez-Hernandez T. Autophagy. 2019 Oct. 2:1-17. doi: 10.1080/15548627.2019.1668606.
- Changes in Dendritic Spine Density and Inhibitory Perisomatic Connectivity onto Medium Spiny Neurons in L-Dopa-Induced Dyskinesia. Gomez G, Escande MV, Suarez LM, Rela L, Belforte JE, Moratalla R, Murer MG, Gershanik OS, Taravini IRE. Mol Neurobiol. 2019 Sep. 56(9):6261-6275. doi: 10.1007/s12035-019-1515-4.
- A collection of integration-free iPSCs derived from Parkinson’s disease patients carrying mutations in the GBA1 gene. Rodríguez-Traver E, Díaz-Guerra E, Rodríguez C, Fernández P, Arenas F, Araúzo-Bravo M, Orera M, Kulisevsky J, Moratalla R, Vicario C. Stem Cell Res. 2019 Jul. 38:101482. doi: 10.1016/j.scr.2019.101482.
- Optostimulation of striatonigral terminals in substantia nigra induces dyskinesia that increases after L-DOPA in a mouse model of Parkinson’s disease. Keifman E, Ruiz-DeDiego I, Pafundo DE, Paz RM, Solís O, Murer MG, Moratalla R. Br J Pharmacol. 2019 Jul. 176(13):2146-2161. doi: 10.1111/bph.14663.
- Genetic Knockdown of mGluR5 in Striatal D1R-Containing Neurons Attenuates L-DOPA-Induced Dyskinesia in Aphakia Mice. García-Montes JR, Solís O, Enríquez-Traba J, Ruiz-DeDiego I, Drucker-Colín R, Moratalla R. Mol Neurobiol. 2019 Jun. 56(6):4037-4050. doi: 10.1007/s12035-018-1356-6.
- Genetic enhancement of Ras-ERK pathway does not aggravate L-DOPA-induced dyskinesia in mice but prevents the decrease induced by lovastatin. Ruiz-DeDiego I, Fasano S, Solís O, Garcia-Montes JR, Brea J, Loza MI, Brambilla R, Moratalla R. Sci Rep. 2018 Oct. 8(1):15381. doi: 10.1038/s41598-018-33713-3.
- Striatal Reinnervation Process after Acute Methamphetamine-Induced Dopaminergic Degeneration in Mice. Granado N, Ares-Santos S, Tizabi Y, Moratalla R. Neurotox Res. 2018 Oct. 34(3):627-639. doi: 10.1007/s12640-018-9925-z.
- Dopamine receptors: homomeric and heteromeric complexes in L-DOPA-induced dyskinesia. Solís O, Moratalla R. J Neural Transm (Vienna). 2018 Aug. 125(8):1187-1194. doi: 10.1007/s00702-018-1852-x.
- Differential Synaptic Remodeling by Dopamine in Direct and Indirect Striatal Projection Neurons in Pitx3-/- Mice, a Genetic Model of Parkinson’s Disease.
- Suarez LM, Alberquilla S, García-Montes JR, Moratalla R. J Neurosci. 2018 Apr.38(15):3619-3630. doi: 10.1523/JNEUROSCI.3184-17.2018.
- Cholesterol and multilamellar bodies: Lysosomal dysfunction in GBA-Parkinson disease. García-Sanz P, Orgaz L, Fuentes JM, Vicario C, Moratalla R. Autophagy. 2018. 14(4):717-718. doi: 10.1080/15548627.2018.1427396.
- The importance of cholesterol in Parkinson’s disease. García-Sanz P and Moratalla R. Movement Disorders. 2018 Feb. 33(2):343-344. doi: 10.1002/mds.27251.
Contacto
Where to find us
Basal Ganglia Neurobiology Laboratory (Laboratorio B-01)
Instituto Cajal CSIC. Avda. Doctor Arce, 37. 28002. Madrid
Call us
Office phone / laboratory phone
+34 915 854 705 / +34 915 854 726
Write us
Others
Last national projects awarded (2018-2022)
- Molecular mechanisms of early non-motor symptoms in Parkinson’s disease: role of asyn accumulation, GBA and lipid metabolism. Ref: PID2019-111693RB-I00.
Entidad financiadora: Ministerio de Ciencia e Innovación.
01/01/2020-30/06/2023. Dotación: 366.630 €.
- Efecto de las mutaciones del gen glucocerebrosidasa-1 en neuronas derivadas de células iPS de enfermos de Parkinson. Rescate del fenotipo y trasplante celular. Área: Terapia celular en enfermedades neurodegenerativas. Entidad: Fundación Ramón Areces, IP: Carlos Vicario
02/03 2017-01/03/2020. Dotación: 120.000 €
- Estudio del consumo de metanfetamina en la adolescencia como factor de riesgo para la adición y la vulnerabilidad dopaminérgica en el adulto: papel de la glía y del glutamato Ref: PNSD 2016I033.
Entidad financiadora: Ministerio de Sanidad, Servicios Sociales e Igualdad y Plan Nacional Sobre Drogas.
01/01/2017-31/12/2019. Dotación: 68.943 €.
- Bases moleculares de la plasticidad sináptica estriatal en las disquinesias y el trastorno de control de impulsos inducidos por L-DOPA en la enfermedad de Parkinson. Ref: SAF2016-78207-R.
Entidad financiadora: Ministerio de Economía, Industria y Competitividad.
01/01/2017-31/12/2019. Dotación: 350.000 €.
- Potencial patológico de los astrocitos: Una nueva perspectiva en la enfermedad de Alzheimer. Ref: 2015-2/02, formado por 5 grupos de investigación.
Entidad financiadora: CIBERNED, Instituto de Salud Carlos III.
2016-2017. Dotación total 350.000 €, 70.000 € grupo R. Moratalla.
Coordinador: Joan Comella. Investigador principal: R. Moratalla.
Last international projects awarded (2018-2022)
- Comorbidity mechanisms of anxiety and Parkinson’s disease (AND-PD), grant agreement n° 848002. Entidad financiadora: Unión Europea, Programa: Horizon 2020, convocatoria: H2020-SC1-BHC-2018-2020
Coordinador del Proyecto: Rosario Moratalla
01/01/2020-30/06/2024 Dotación IP: 805.000 €
- Development of a new in vivo radiotracer for αsynuclein. PCIN-2015-098
Entidad financiadora: Unión Europea, ERANET Euronanomed, Ministerio de Economía y Competitividad.
01/10/2015-30/09/2019. Dotación: 115.000€.
Coordinador: Mireille Dumoulin. Investigador principal: R. Moratalla.
Latest doctoral theses 2017-2023
- Adrián Sanz Magro. Bases moleculares de la neurodegeneración catecolaminérgica y su relación con los síntomas de ansiedad y depresión en la enfermedad de Parkinson. Marzo 2023. Universidad Autónoma de Madrid. Sobresaliente Cum Laude.
- Samuel Alberquilla. Regulación dopaminérgica de la plasticidad estructural y sináptica de las neuronas estriatales de proyección. Diciembre 2022. Universidad Autónoma de Madrid. Sobresaliente Cum Laude.
- Ettel Keifman Mecanismos involucrados en la generación de discinesias inducidas por L-DOPA en un modelo experimental de la enfermedad de Parkinson Diciembre, 2019. Universidad Autónoma de Buenos Aires (Argentina). Sobresaliente Cum Laude
- Lorena Orgaz Gordillo Implicación de las mutaciones N370S y L444P en el gen GBA1 en la desregulación de la homeostasis celular y su relación con la enfermedad de Parkinson. 2019. Universidad Autónoma de Madrid. Sobresaliente Cum Laude.
- Óscar Solís Castrejón Efecto de la inactivación genética de los receptores dopaminérgicos D3 en el desarrollo y expresión de las disquinesias. Julio, 2017. Universidad Autónoma de Madrid. Sobresaliente Cum Laude. Premio Alberto Rábano 2018. Fundación Romanillos. (Premio que se otorga a la mejor tesis doctoral sobre neurociencias básicas y clínicas, neurología, neurocirugía, neuropatología, neurofisiología o psiquiatría, defendida en alguna Universidad Española durante 2017).
TFM and TFG (2019-2022)
- Iris del Val. Trabajo Fin de Máster. Master en Neurociencias. Facultad de Biologia. Universidad Complutense de Madrid. Septiembre 2022.
- Cristina Gonzalez Dragado. Trabajo Fin de Máster. Master en Neurociencias. Facultad de Medicina. Universidad Autónoma de Madrid. Septiembre 2022.
- Viktoriya Kaloyanova Karaivanova Cambios en la longitud y arborización dendrítica del estriado een el tratamiento con L-Dopa en eun modelo murino de la enfermedad de Parkinson. Trabajo Fin de Máster. Master en Neurociencias. Facultad de Medicina. Universidad Autónoma de Madrid. Septiembre 2020
- Guiomar Rodríguez Periñan Alteraciones moleculares y funcionales inducidas por la agregación de α-sinucleína en el núcleo dorsal del rafe y su impacto en la actividad β-glucocerebrosidasa. Trabajo Fin de Máster. Master en Neurociencias. Facultad de Medicina. Universidad Autónoma de Madrid. Septiembre 2020.
- Elena Torres Campos Impacto celular y funcional de la acumulación de α‐sinucleína en el locus coeruleus y su correlación con la ansiedad en el modelo Glong de la enfermedad de Parkinson. Trabajo Fin de Máster. Master en Neurociencias. Facultad de Medicina. Universidad Autónoma de Madrid. Septiembre 2020.
- Iris Menéndez Fernández Impacto de la mutación L444P en el gen GBA1 en la integridad de la red mitocondrial y la distribución lisosomal en la enfermedad de Parkinson. Trabajo Fin de Máster. Master en Neurociencias. Facultad de Biología. Universidad Complutense de Madrid. Octubre 2019.
- Mónica Gómez Benito Caracterización de los mecanismos moleculares de la Enfermedad de Parkinson tras la infección con AAV de α-sinucleína humana mutada en ratón. Trabajo Fin de Máster. Master en Neurociencias. Facultad de Medicina. Universidad Autónoma de Madrid. Septiembre 2019.
- Marta Posada Gracia Alteración neuronal del núcleo dorsal del rafe en un modelo genético de la enfermedad de Parkinson. Trabajo Fin de Máster. Master en Bioquímica, Biología Molecular y Biomedicina. Facultad de Ciencias Químicas. Universidad Complutense de Madrid. Julio 2019.
- Irene Costa Laparra Estudio de la red mitocondrial y la autofagia en un modelo celular paciente-específico de la enfermedad de Alzheimer. Trabajo Fin de Máster. Master en Neurociencias. Facultad de Biología. Universidad Complutense de Madrid. Julio 2019.
Books
- Moratalla R., Sanz-Magro A., Granado N. (2021) Amino-Cupric-Silver (A-Cu-Ag) Staining to Detect Neuronal Degeneration in the Mouse Brain: The de Olmos Technique. In: Llorens J., Barenys M. (eds) Experimental Neurotoxicology Methods. Neuromethods, vol 172. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-1637-6_1
- Moratalla R, Solís O, Suarez LM. Morphological Plasticity in the Striatum Associated with Dopamine Dysfunction. Incluído en: Handbook of Basal Ganglia Structure and Function 2e. 2017. H Steiner and KY Tseng (Eds.), Elsevier Inc., San Diego: Academic Press. 755-770. ISBN: 9780128022061
- Moratalla R, Ares-Santos S, Granado N. Neurotoxicity of Methamphetamine. Incluído en: Handbook of neurotoxicity. 2014. RM Kostrzewa (Ed.), Editorial Springer-Verlag, New York. 2207-2230. ISBN 978-1-4614-7458-6. DOI: 10.1007/978-1-4614-5836-4
- Ares-Santos S, Granado N, Moratalla R. Neurobiology of Methamphetamine. Incluído en: Biological Research on Addiction: Comprehensive Addictive Behaviours and Disorders. 2013. Miller PM (Ed.), Elsevier Inc., San Diego: Academic Press. 579-591. ISBN: 9780123983350.
Teaching
- Neurophysiopathology of Parkinson’s disease. Master in Neurosciences from the Complutense University of Madrid.
- Neurobiological Bases of Drug Addiction. Master in Neurosciences from the Autonomous University of Madrid. Cajal Institute, Madrid.
- Neurobiology of brain aging and diseases of the nervous system. Master in Neurosciences from the Autonomous University of Madrid.

Neuroscience Research Center dependent on the CSIC. Founded in 1920 and initially directed by Santiago Ramón y Cajal. World reference in the study of the brain. Custodian of the Cajal Legacy.
Activities
