ACE OF SWORDS
MICROGLÍA
Ace of swords: microglia, the defenders of nervous tissue

Introducing our Ace of Swords: microglia. A “micro” cell due to its size, but a “macro” cell due to its defense powers of the central nervous system.
As its name suggests, microglia are the smallest glial cells in our central nervous system. In fact, it is the smallest of the cells that we can find in the brain (cerebrum, cerebellum and medulla oblongata) and the spinal cord.
Until 1920, microglia cells had been described as part of the “third element” of nervous tissue, but from that year on, the disciple of Santiago Ramón y Cajal and Nicolás Achúcarro, Pío del Río-Hortega, decided that they were independent cells and gave them the name by which we know them today [1]. In 1925, Ramón y Cajal wrote:
«Pío del Río-Hortega is credited with having found a special method capable of completely showing, down to its finest ramifications, normal and pathological brain microglia. Thanks to this precious technical contribution, applicable to man and mammals, it has been shown that the Stäbchenzellen, the “interstitial” cells of Achúcarro and all the corpuscles full of granulations, known by the names of Gitterzellen, Füllzellen and Abräumzellen, are no more “than simple varieties of normal microglia that would be endowed with a surprising faculty of emigration and phagocytic power.” [2]
Human beings have almost as many microglia cells as neurons in the brain, although their number varies depending on whether we are talking about physiological, inflammatory, or pathological conditions. They are not distributed evenly, but the proportion of microglia in different brain regions ranges from 0.5% to 16.6% of the total cells [3].
They are very versatile glial cells, since both their shape and their structure and distribution in the nervous system vary depending on the functions they are developing at any given time, the place where they are located and the chemical signals they receive from the cells. that surround them (whether these are other glial cells, neurons or infiltrates of cells foreign to the nervous system in pathological situations). They are therefore very dynamic cells.
Each of the different states of microglia is known in our field of neuroscience as a “phenotype.” Although there are many different phenotypes, under the microscope two types of cells can be differentiated simply by the shape they have: activated cells (reactive microglia) and resting cells (non-reactive microglia).
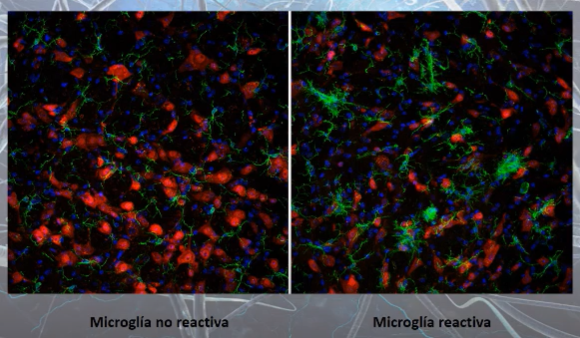
Confocal microscopy images of microglia cells (green color) with their non-reactive (left) and reactive (right) phenotypes
(source https://youtu.be/1SZ2HVnGwIk?list=PLEU_4DYTRsCHPRnT2mlufUmpb_J0KlwmY&t=1623)
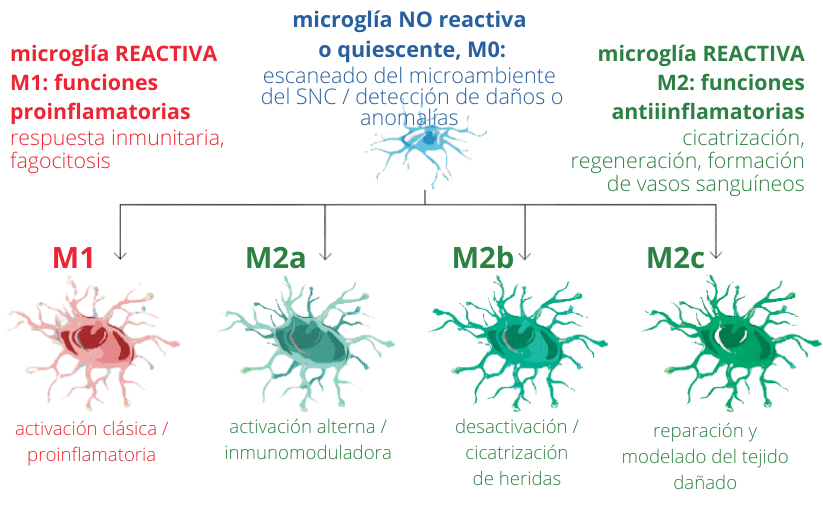
Microglia activation profiles. Depending on the stimulus, microglia can acquire different types of activation and will therefore have various functions.
(adapted from MARTÍNEZ TAPIA et al. (2018). Neuroinflammation: The Ying-Yang of Neuroimmunology. Revista De La Faculty of Medicine (Mexico), 61(5), 44-53)
At rest, microglia cells have a rounded central area, from which multiple projections emerge, resembling the legs of a spider. However, when microglial cells activate (become reactive), these projections disappear and the central part becomes larger, taking on a fried egg shape [4].
In reality, microglia cells are not nerve cells (such as neurons, astrocytes or oligodendrocytes), but are cells of the immune system (they are derived from precursor cells of the bone marrow, of mesodermal origin) that infiltrate the brain and spinal cord during embryonic development. So that? Well, to be as close as possible to dangers that could damage the central nervous system and, if they appear, respond quickly to any disturbance in the system.
Under physiological conditions, microglial cells are constantly “scanning” the environment around them with extensions that extend and retract to verify that the cells around them are in an optimal state. It is estimated that they travel through the brain in about six hours. These projections also allow them to establish contact with different synapses between neurons to verify that they are working.
In the event that the cells are not in an optimal state or the synapses do not function, the microglia cells undergo different degrees of activation in which their shape changes and the extensions become shorter, until they reach the state of maximum activation. in which they take a rounded shape without projections (like a fried egg). In this state is when they can exert macrophage functions to eliminate cellular debris (debris) and dead neurons from nervous tissue, thanks to a process called “phagocytosis” and which is nothing other than “eating” these remains. These changes, which we are able to see in the shapes, are the mirror of what is happening within the microglia and their functional state [5].
When faced with any disturbance in the environment that surrounds the microglia cells, they initially respond by releasing chemical substances that have two functions: eliminate the disturbance and attract other cells to the area where the problem is happening, in order to deal with it. This process is known as “neuroinflammation.”
Generally, neuroinflammation is a short-term process, in which what is altering homeostasis (maintenance of the composition and properties of the internal environment of an organism) is eliminated and microglia cells phagocytize the remains, releasing anti-inflammatory substances (which eliminate inflammation), and return to their resting state.
The so-called “synapse pruning” occurs when microglial cells detect that there are contacts between neurons that are not working or that are redundant, and decide to phagocytose them so that information flows more efficiently and is not lost in useless connections. The goal of synaptic pruning is therefore to achieve a precise connection of brain circuits.
All microglia cells express receptors on their surface that we can use to mark them and view them under a microscope. The most used are Iba-1 and CD11b, but in addition to these markers, there are others that serve to recognize each of the microglia phenotypes. These other markers can be: membrane receptors (for example to see those that phagocytize more, or those that interact more with other cells), enzymes inside the cell (which give an idea of what biochemical pathways—pro-inflammatory or anti-inflammatory—are being used. activating in a certain situation), or other chemical substances (such as senescence markers that will tell us if the cells are working well or not). Together, all these markers are what will allow us to know what is happening.
In pathological situations, when we study the affected region, several microglia phenotypes are generally found in the same area. That is, we see pro-inflammatory microglia, whose purpose is to eliminate damage, and anti-inflammatory microglia, whose purpose is to control and resolve inflammation before it is harmful to nervous tissue. In pathological situations, as the disease progresses, there is an imbalance of these phenotypes and the microglia response is no longer efficient. The proinflammatory phenotype or a senescent phenotype that does not respond then appears more frequently.
It has been seen that microglia are essential in the development of the human nervous system, since in these initial stages, cell division occurs in waves where it is crucial to eliminate excess cells so that all the nervous structures that we know in the adult nervous system are formed and function properly. In fact, it has been observed that a malfunction or microglia in these stages could be related to the appearance of diseases such as autism spectrum and behavioral disorders in children.
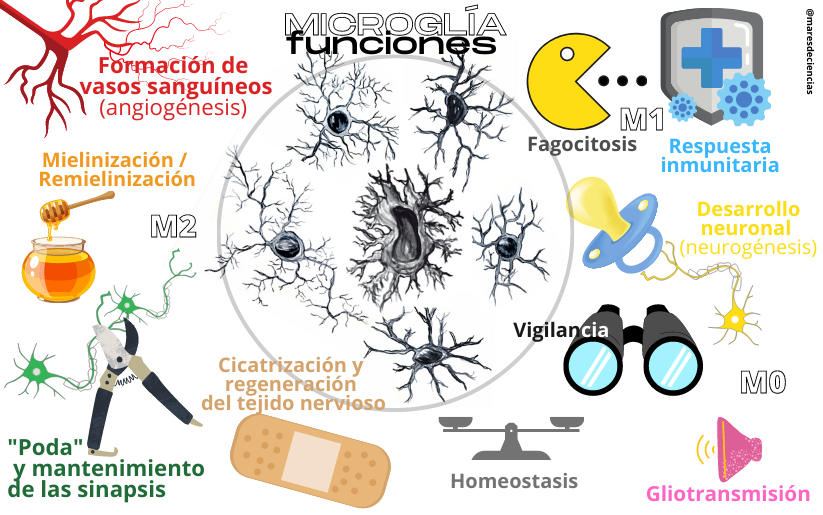
Functions of microglia
(drawings of microglia cells: Natalia Yanguas Casás)
Microglia also play a crucial role in all neurodegenerative diseases, as they are mostly accompanied by neuroinflammation.
As we have explained before, neuroinflammation is a physiological process whose purpose is to cope with some disturbance in the environment to protect the individual. However, if this process is not resolved and becomes chronic, the inflammatory mediators released by microglia cells activate other cells (such as astrocytes, microglia and even cells that have been recruited from outside the nervous system) and situations are generated. toxic that can lead to cell death. Furthermore, as we age, microglia can become senescent and become unable to resolve the alterations they perceive in their environment with the same efficiency as in younger stages.
In diseases such as multiple sclerosis, in addition to the chronic neuroinflammation process, deficient phagocytosis of myelin by microglia cells has been described, which generates an inflammatory feedback process that is increasingly difficult to resolve, until it reaches a point at which the system cannot cope and the disease progresses [6].
Something similar also occurs in Alzheimer’s disease, in which it has been found that microglia concentrate around amyloid plaques [7], and that they activate and divide in these areas, but are not capable of phagocytosing the plaques. and eliminate them [8].
In the case of Parkinson’s disease, deficits in the microglial response affect the development of the disease through the process of inflammation and deficient phagocytosis of α-synuclein, among others [9].
Certain alterations in microglia functions leading to the appearance of aberrant synapses are also known to contribute to the pathophysiology of epilepsy [10].
These are just some examples of diseases in which alterations in microglia responses contribute to their development. But as we have told you, they are involved in all of them, since they control neuroinflammation, phagocytosis and the formation of synapses.
Did you know before reading this how essential this cell is for our brain to function properly?
Have you been curious and want to know more?
NOTES AND SOURCES CITED
[1] At the beginning of the 20th century, the work and stains used by Ramón y Cajal and Achúcarro made it possible to distinguish in the neuroglia protoplasmic astrocytes of the gray matter (short-radiation glia), fibrous astrocytes (long-radiation glia) and a “third element” (in Cajal’s terminology) that could not be distinguished precisely, made up of “adendrite elements.” Pío del Río-Hortega managed to improve the staining techniques that he had learned with Achúcarro, thanks to the silver carbonate method, and saw much more than his teachers; He published it in a 1919 work in which he coined the term “microglia”, whose title is very significant: “The third element of the nervous centers.” I Normal microglia. II Intervention of microglia in pathological processes. (Rod cells and granulo-adipose bodies). III Probable nature of microglia”, Bull. Soc. Esp. Biol., 9, 69-129. In: CORTÉS GABAUDAN, Francisco (2009). Microglia, a 20th century Spanish contribution to the medical vocabulary. Panace@. Vol.
[2]Works of the Biological Research Laboratory, 23 (1925): 157-216].
[3] LAWSON, L.J., V.H. PERRY, S. GORDON (1992). Turnover of resident microglia in the normal adult mouse brain. Neuroscience, Volume 48, Issue 2, 1992: pp. 405-415 https://doi.org/10.1016/0306-4522(92)90500-2
[4] LAWSON, L.J., V.H. PERRY, P. DRI and S. GORDON (1990). Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39(1):151-70. https://doi.org/10.1016/0306-4522(90)90229-W
[5] DAVIS, E.J., T.D. FOSTER and W.E. THOMAS (1994). Cellular forms and functions of brain microglia. Brain Res Bull. 1994;34(1):73-8. https://doi.org/10.1016/0361-9230(94)90189-9
[6] GUERRERO BROOKE, L. and Nancy L. SICOTTE (2020). Microglia in Multiple Sclerosis: Friend or Foe? Frontiers in Immunology, vol. eleven,
https://www.frontiersin.org/article/10.3389/fimmu.2020.00374
[7] Amyloid plaques are the result of the accumulation of a protein in the extraneuronal space characteristic of people affected by Alzheimer’s disease, preventing a correct connection between neurons and causing their degeneration.
[8] HANSEN, David V., Jesse E. HANSON and Morgan SHENG (2018). Microglia in Alzheimer’s disease. J. Cell Biol (Special Collection: Neurogeneration and neuroinflammation) vol 217 (2): 459-472 https://doi.org/10.1083/jcb.201709069
[9] HO MS. (2019). Microglia in Parkinson’s Disease. In: Verkhratsky A., Ho M., Zorec R., Parpura V. (eds) Neuroglia in Neurodegenerative Diseases. Advances in Experimental Medicine and Biology, vol 1175. Springer, Singapore. https://doi.org/10.1007/978-981-13-9913-8_13
[10] VICTOR TR and SE. TSIRKA (2020). Microglial contributions to aberrant neurogenesis and pathophysiology of epilepsy. Neuroimmunol. Neuroinflammation 2020;7:234-47. http://dx.doi.org/10.20517/2347-8659.2020.02
OTHER LINKS AND DOCUMENTS
Cajal Institute YouTube Channel
Neuroscience For Dummies, Natalia Yanguas Casás.
https://Youtu.Be/1SZ2HVnGwIk?List=PLEU_4DYTRsCHPRnT2mlufUmpb_J0KlwmY&T=1623
Blog Glial Cells
Microglia https://Celulasgliales.Com/Microglia/
Synapsis EMP YouTube Channel
Microglia https://Www.Youtube.Com/Watch?V=-AV8BJXhyDU
Phagocytosis And Antigen Presentation https://Www.Youtube.Com/Watch?V=MFVcJur-D-S
CRESPO CASTRILLO, Andrea (2019). Effect of the synthetic steroid tibolone on reactive gliosis. Doctoral thesis of the Department of Pharmacology of the Autonomous University of Madrid, directed by Dr. Luis Miguel García Segura and Dr. María Ángeles Arévalo Arévalo (Instituto Cajal, CSIC) http://hdl.handle.net/10486/690490
DEL RÍO-HORTEGA, Pío (1919). Rapid staining of normal and pathological tissues with ammoniacal silver carbonate. Works of the Histopathology Laboratory of the Board for the Expansion of Studies No. 7. Bulletin of the Spanish Society of Biology, Vol. . 235-243 https://arbor.revistas.csic.es/index.php/arbor/article/view/432/434
DEL RÍO-HORTEGA, Pío (1924). What should be understood by the Third Element of the nervous centers. Bulletin of the Spanish Society of Biology, Vol. 245-248
https://arbor.revistas.csic.es/index.php/arbor/article/view/433/435
MEDEROS CRESPO, Sara (2019). Astrocyte-interneuron communication and information processing in neuronal networks. Doctoral Thesis directed by Dr. Gertrudis PEREA PARRILLA, from the Cajal Institute (CSIC). Complutense University of Madrid. Faculty of Biological Sciences, Department of Biochemistry and Molecular Biology. 198 pp. https://digital.csic.es/handle/10261/210592
PÉREZ CAPOTE, Kamil (2006). Response of glial cells to neuronal damage in vitro. Introduction (55 pp). Doctoral Thesis. University of Barcelona. https://digital.csic.es/bitstream/10261/91949/4/1_INTRODUCCION.pdf
SIERRA, Amanda, PAOLICELLI Rosa C., and KETTENMANN H. (2019). One Hundred Years of Microglia: Milestones in a Century of Microglial Research. Trends in Neurosciences, November, Vol. 42, No. 11, pp. 778-792. https://doi.org/10.1016/j.tins.2019.09.004
KETTENMANN, H.U.K. HANISCH, M. NODA & A. VERKHRATSKY (2011). Physiology Of Microglia. Physiological Reviews, 91(2), 461-553. https://Doi.Org/10.1152/Physrev.00011.2010
MARTÍNEZ TAPIA, Ricardo Jesús, Francisco ESTRADA-ROJO, Alonso Alejndro HERNÁNDEZ-CHÁVEZ, Antonio BARAJAS MARTÍNEZ, Santiago ISLAS ESCOTO, Luz NAVARRO AND Anahí CHAVARRÍA (2018). Neuroinflammation: The Ying-Yang of Neuroimmunology. Revista De la Faculty of Medicine (Mexico), 61(5), 44-53. Http://Www.Scielo.Org.Mx/Scielo.Php?Script=Sci_arttext&Pid=S0026-17422018000500044&Lng=Es&Tlng=Es.
NAKAJIMA, K. & S. KOHSAKA (2001). Microglia: Activation And Their Significance In The Central Nervous System. The Journal Of Biochemistry, 130(2), 169-175. https://Doi.Org/10.1093/Oxfordjournals.Jbchem.A002969
YANGUAS CASÁS, Natalia (2015). Effect of tauroursodeoxycholate on the regulation of acute neuroinflammation. Doctoral Thesis, according to the work carried out under the direction of Dr. Lorenzo Romero Ramírez and Prof. Manuel Nieto Sampedro in the Department of Functional and Systems Neurobiology of the Cajal Institute (CSIC), Autonomous University of Madrid (UAM) , Department of Biochemistry, Molecular Biology, Biomedicine and Biotechnology. 187 pp.
AND TO CHECK IF YOU HAVE UNDERSTOOD EVERYTHING WE HAVE TOLD YOU ABOUT MICROGLIA, here is a small interactive questionnaire.
